Advertisements
Advertisements
Question
Answer the following.
Write a reaction to bring about the following conversions: Aniline into sulphanilic acid?
Solution
Sulfonation: Aniline reacts with concentrated sulphuric acid to form anilinium hydrogen sulphate which on heating with sulphuric acid at 453-473K produces p-aminobenzene sulphonic acid (sulphanilic acid) as a major product.
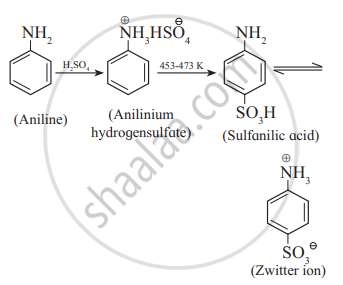
Sulphanilic acid exists as a salt; called dipolar ion or zwitter ion. It is produced by the reaction between an acidic group and a basic group present in the same molecule.
RELATED QUESTIONS
Answer the following
Write a reaction to convert Aniline into p-bromoaniline.
Answer the following.
Write a reaction to bring about the following conversions: Aniline into p-nitroaniline
Aniline on reaction with bromine water produces ________________
The following compound is a/an ____________.
Which of the following molecules form a Zwftter ion?
Identify the product 'B' in the following reaction.
\[\ce{Benzamide ->[Br2/KOH] A ->[Br2 water][298 K] B}\]
Which of the following molecule can form a zwitter ion?
Identify the CORRECT statements.
(I) Aniline reacts with bromine water at room temperature to give o-bromoaniline.
(II) Aniline undergoes Friedel Craft's reaction using aluminium chloride.
(III) Direct nitration of aniline yields, a mixture of ortho, meta and para nitro aniline.
(IV) Amino group is ortho and para directing and powerful ring activating group.
Identify the product obtained when chlorobenzene is heated with ammonia and Cu2O at 473 K under pressure.
Which of the following groups increases the basic strength of substituted aniline?
The major product obtained when aniline undergoes sulphonation using cone. sulphuric acid at 453-473 K is:
Aniline on reaction with Bromine water at room temperature gives ____________.
When propanamide is treated with bromine and aqueous sodium hydroxide, the compound formed is ____________.
What is the action of aqueous bromine on aniline?
