Advertisements
Advertisements
Question
Write reactions to prepare ethanamine from Acetonitrile.
Solution
Acetonitrile on Mendius reduction yields ethanamine.
\[\ce{\underset{\text{Acetonitrile}}{CH3 - C ≡ N} + 4[H] ->[Na/C2H5OH][or LiAlH] \underset{\text{Ethanamine}}{CH3 - CH2 - NH2}}\]
APPEARS IN
RELATED QUESTIONS
Write a short note on Hoffmann bromamide degradation.
Illustrate the following reaction giving suitable example in each case:Gabriel phthalimide synthesis
Give the structures of A, B and C in the following reactions :
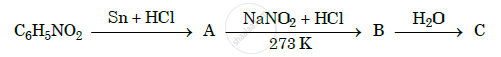
Write short notes on the following Hofmann’s bromamide reaction
Give the structures of A, B and C in the following reaction:

Give the structures of A, B and C in the following reaction:
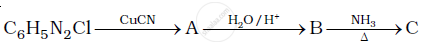
Give the structures of A, B and C in the following reaction:
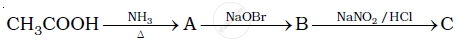
Why cannot aromatic primary amines be prepared by Gabriel phthalimide synthesis?
Arrange the following in the increasing order of their pKb values:
C6H5NH2, C2H5NH2, C6H5NHCH3
Give the structures of A, B and C in the following reactions :
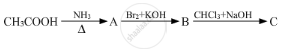
Write structures of compounds A and B in each of the following reactions: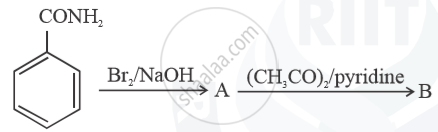
Choose the most correct option.
Which of the following compounds will dissolve in aqueous NaOH after undergoing reaction with Hinsberg reagent?
Answer in one sentence.
Which amide does produce ethanamine by Hofmann bromamide degradation reaction?
Answer the following
Identify A and B in the following reactions.
\[\ce{C6H5CH2Br->[alco.][KCN]A ->[Na/ethanol]B.}\]
Answer the following
Explain Gabriel phthalimide synthesis.
Write reactions to bring about the following conversions.
Acetamide to Ethylamine
Write reactions for the preparation of ethanamine using Gabriel phthalimide synthesis.
Why cannot aniline be prepared by Gabriel phthalimide synthesis?
Identify the product obtained when benzyl chloride undergoes ammonolysis in presence of excess ammonia followed by the reaction with two moles of methyl iodide.
Which of the following amines exhibits maximum degree of intermolecular hydrogen bonding?
What product is formed when \[\ce{R - C ≡ N}\] is hydrolysed?
Which of the following amines forms a clear solution when treated with benzene sulphonyl chloride and excess of potassium hydroxide?
Identify 'A' and 'B' in the following conversions.
\[\ce{CH3 - I ->[Alc. KCN][\Delta] A ->[Na/C2H5OH] B}\]
In aqueous phase the order of basic strength of alkylamine is ______.
Which of the following reagents is used in Mendius reduction reaction of alkyl cyanide?
Identify product B in the following reaction.
\[\ce{Aniline ->[NaNO2][HCl] A ->[KI] B}\]
Which of the following does NOT give carbylamine test?
Which of the following reactions does NOT yield an amine?
Quaternary ammonium salt is formed:
Given below are two statements labelled as Assertion (A) and Reason (R).
Assertion (A): Alkyl halides are insoluble in water.
Reason (R): Alkyl halides have halogen attached to sp3 hybrid carbon.
Select the most appropriate answer from the options given below:
Benzylamine may be alkylated as shown in the following equation:
\[\ce{C6H5CH2NH2 + R - X -> C6H5CH2NHR}\]
Which of the following alkylhalides is best suited for this reaction through SN1 mechanism?
Reduction of nitrobenzene by which of the following reagent gives aniline?
(i) \[\ce{Sn/HCl}\]
(ii) \[\ce{Fe/HCl}\]
(iii) \[\ce{H2 - Pd}\]
(iv) \[\ce{Sn/NH4OH}\]
Which of the following reactions are correct?
(i)
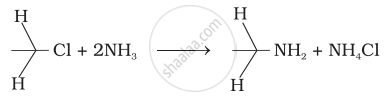
(ii)
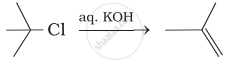
(iii)
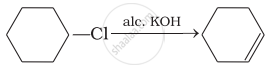
(iv)
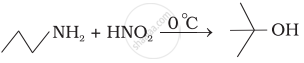
Identify A and B in the following reaction.
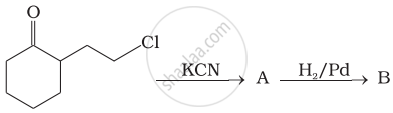
Write following conversions:
nitrobenzene `->` acetanilide
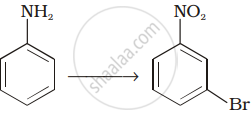
How will you carry out the following conversion?
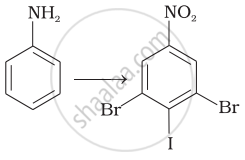
How will you carry out the following conversions?
Assertion: Only a small amount of \[\ce{HCl}\] is required in the reduction of nitro compounds with iron scrap and \[\ce{HCl}\] in the presence of steam.
Reason: \[\ce{FeCl2}\] formed gets hydrolysed to release \[\ce{HCl}\] during the reaction.
The Gabriels' phthalimide synthesis is used in the synthesis of
A primary amine is formed by an amide on treatment with bromine and alkali. The primary amine has
Reduction of nitro alkanes yields which compound?
Acetamide and ethyl amide can be distinguished by reacting with.
A compound 'A' on reduction with iron scrap and hydrochloric acid gives compound 'B' with molecular formula C6H7N. Compound 'B' on reaction with CHCl3 and alcoholic KOH produces an obnoxious smell of carbylamine due to the formation of 'C'. Identify 'A', 'B' and 'C' and write the chemical reactions involved.
Which of the following compound gives pink colour on reaction with phthalic anhydride in cone. H2SO4 followed by treatment with NaOH?
Methyl amine on reaction with chloroform in the presence of NaOH gives ______.
- Phenyl methenamine
- N, N - Dimethylaniline
- N - Methyl aniline
- Benzenamine
Choose the correct order of the basic nature of the above amines.
Write the name of the product formed by the action of LiAlH4/ether on acetamide.
Write a short note on the following:
Ammonolysis
Assertion: Amimonolysis of alkyl halides involves the reaction between alkyl halides and alcoholic ammonia.
Reason: Ammonolysis of alkyl halides produces secondary amines only.
