Advertisements
Advertisements
Question
Choose the most correct option.
Which type of amine does produce N2 when treated with HNO2?
Options
Primary amine
Secondary amine
Tertiary amine
Both primary and secondary amines
Solution
Primary amine
APPEARS IN
RELATED QUESTIONS
In the following
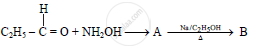
The compound ‘B’ is _______.
(A) Propan–1–amine
(B) Propan–2–amine
(C) Isopropylamine
(D) Dimethylamine
Give reasons for the following:
CH3NH2 is more basis than C6H5NH2.
Isobutylamine is an example of ______.
Choose the most correct option.
Carbylamine test is given by ____________.
What is the action of nitrous acid on the following compounds?
Isopropyl amine
How are amines classified depending on the functional group? Give one example of each class of amines.
A tertiary amine is an organic compound ____________.
Which of the following amines is most basic in nature in aqueous phase?
Assertion: Acetamide on reaction with KOH and bromine gives acetic acid.
Reason: Bromine catalyses hydrolysis of acetamide.
Which one of the following is most basic?
IUPAC name for the amine is:
\[\begin{array}{cc}
\phantom{.}\ce{CH3}\\
|\phantom{..}\\
\ce{CH3 - N - C - CH2 - CH3}\\
\phantom{.}|\phantom{.....}|\phantom{........}\\
\phantom{}\ce{CH3}\phantom{..}\ce{C2H5}\phantom{....}
\end{array}\]
Write a short note on the following.
Gabriel phthalimide synthesis
Write a short note on the following.
Mustard oil reaction
Account for the following.
pKb of aniline is more than that of methylamine.
Account for the following.
Gabriel phthalimide synthesis is preferred for synthesising primary amines.
Arrange the following.
In decreasing order of basic strength in gas phase (C2H5)NH2, (C2H5)NH, (C2H5)3N and NH3.
Arrange the following.
In decreasing order of the pKb values C2H5NH2, C6H5NHCH3, (C2H)2NH and CH3NH2.
Arrange the following.
In decreasing order of basic strength
![]()
How will you prepare propan-1-amine from propanamide?
Identify A, B and C.
\[\ce{CH3 - NO2 ->[LiAlH4] A ->[2CH3CH2Br] B ->[H2SO4] C}\]
How will you convert diethylamine into N, N-diethyl acetamide?
How will you convert diethylamine into N-nitrosodiethylamine?
(C2H5)2CHNH2. The following amine can be classified as:
The following amine can be classified as (C2H5)2NH:
The main product is formed by treating an alkyl or benzyl halide with excess ammonia ____________.
Arrange the following in the increasing order of their basic strength:
C2H5NH2, C6H5NH2, (C2H5)2NH
Complete the following reaction.
Classify the following amine as primary, secondary or tertiary:

Which among the following is the strongest Bronsted base?
Assertion A: Aniline on nitration yields ortho, meta and para nitro derivatives of aniline.
Reason R: Nitrating mixture is a storng acidic mixture.
In the light of the above statements, choose the correct answer from the options given below:
Arrange the increasing order of solubility in water.
\[\ce{C2H5Cl, C2H5NH2, C2H5OH}\]
Write short notes on the following
Ammonolysis
Write a short note on the following.
Ammonolysis
Name the distinguishing test for differentiating 1° amine from 2° and 3° amine.
Write short note on the following.
Ammonolysis
Write a short note on the following.
Ammonolysis
Account for the following:
Aniline does not undergo Friedel-Crafts reaction.
Write short note on the following.
Ammonolysis
Write a short note on the following.
Ammonolysis
