Advertisements
Advertisements
Question
Arrange the following.
In decreasing order of basic strength
![]()
Solution
Aliphatic amines are more basic than aromatic amines. Therefore CH3CH2NH2 and CH3NH2 are more basic. Among the ethylamine and methylamine. ethylamine was experienced more +I effect than methylamine and hence ethylamine is more basic than methylamine.
Nitro group has a powerful electron-withdrawing group and they have both – R effect as well as – I effect. As a result, all the nitro anilines are weaker bases than aniline. In P-nitro aniline
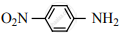
both – R effect and – I effect of the NO2 group decrease the basicity. Therefore decreasing order of basic strength is,
![]()
Ethylamine > Methylamine > Aniline > p-nitro aniline
APPEARS IN
RELATED QUESTIONS
Isobutylamine is an example of ______.
Choose the most correct option.
Which type of amine does produce N2 when treated with HNO2?
The order of basic strength for methyl substituted amines in aqueous solution is ____________.
Account for the following.
pKb of aniline is more than that of methylamine.
Account for the following.
Gabriel phthalimide synthesis is preferred for synthesising primary amines.
How will you convert diethylamine into N, N-diethyl acetamide?
The reaction of NHO2 with 'A' gives quartering ammonium salt. A is which of the following?
\[\begin{array}{cc}
\ce{O}\phantom{.........}\\
||\phantom{.........}\\
\ce{H - \underset{(A)}{C} - NH - CH3}
\end{array}\]
and
\[\begin{array}{cc}
\ce{O}\\
||\\
\ce{CH3 - \underset{(B)}{C} - NH2}
\end{array}\]
are which type of isomers?
Write a short note on the following.
Ammonolysis
Write a short note on the following.
Ammonolysis
