Advertisements
Advertisements
Question
Arrange the following.
Increasing order of basic strength C6H5NH2, C6H5N(CH3)2, (C2H5)2NH and CH3NH2.
Solution
Due to the delocalization of the lone pair of electrons of the N-atom over the benzene ring, all aromatic amines are less basic than alkylamines i.e., CH3NH2. The presence of electron-donating groups (– CH3) on the N-atom increases the basicity of substituted aniline with respect to C6H5NH2.
In (C6H5)NH2, the lone pair of electrons on the N-atom is delocalized over two benzene rings instead of one in C6H5NH2, therefore (C6H5)NH2 is much less basic than C6H5NH2. Combining all the three trends together, the basic strength of the four amines increasing in order.
(C6H5)NH2 < (C2H5)2NH < C6H5N(CH3)2 < CH3NH2
APPEARS IN
RELATED QUESTIONS
In the following
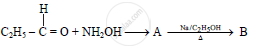
The compound ‘B’ is _______.
(A) Propan–1–amine
(B) Propan–2–amine
(C) Isopropylamine
(D) Dimethylamine
How are amines classified?
Assertion: Acetamide on reaction with KOH and bromine gives acetic acid.
Reason: Bromine catalyses hydrolysis of acetamide.
Write a short note on the following.
Carbylamine reaction
Arrange the following.
In decreasing order of basic strength in gas phase (C2H5)NH2, (C2H5)NH, (C2H5)3N and NH3.
How will you prepare propan-1-amine from propanamide?
Identify A, B and C.
\[\ce{CH3 - NO2 ->[LiAlH4] A ->[2CH3CH2Br] B ->[H2SO4] C}\]
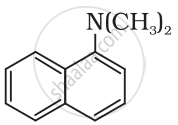
The following amine can be classified as:
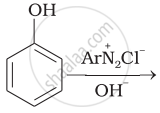
Complete the following reaction.
Write short notes on the following
Ammonolysis
