Advertisements
Online Mock Tests
Chapters
2: p-Block Elements - I
3: p-Block Elements - II
4: Transition and Inner Transition Elements
5: Coordination Chemistry
6: Solid State
7: Chemical Kinetics
8: Ionic Equilibrium
9: Electro Chemistry
10: Surface Chemistry
11: Hydroxy Compounds and Ethers
12: Carbonyl Compounds and Carboxylic Acids
▶ 13: Organic Nitrogen Compounds
14: Biomolecules
15: Chemistry in Everyday Life
![Samacheer Kalvi solutions for Chemistry - Volume 1 and 2 [English] Class 12 TN Board chapter 13 - Organic Nitrogen Compounds Samacheer Kalvi solutions for Chemistry - Volume 1 and 2 [English] Class 12 TN Board chapter 13 - Organic Nitrogen Compounds - Shaalaa.com](/images/chemistry-volume-1-and-2-english-class-12-tn-board_6:5f2b1b2038084cf381bfa42c826a928c.jpg)
Advertisements
Solutions for Chapter 13: Organic Nitrogen Compounds
Below listed, you can find solutions for Chapter 13 of Tamil Nadu Board of Secondary Education Samacheer Kalvi for Chemistry - Volume 1 and 2 [English] Class 12 TN Board.
Samacheer Kalvi solutions for Chemistry - Volume 1 and 2 [English] Class 12 TN Board 13 Organic Nitrogen Compounds Evaluation [Pages 229 - 235]
Choose the correct answer:
Which of the following reagent can be used to convert nitrobenzene to aniline?
Sn/HCl
ZnHg/NaOH
Zn/NH4Cl
All of these
The method by which aniline cannot be prepared is ____________.
degradation of benzamide with Br2/NaOH
potassium salt of phthalimide treated with chlorobenzene followed by hydrolysis with aqueous NaOH solution
reduction of Nitrobenzene with LiAlH4
reduction of nitrobenzene by Sn/HCl
Which one of the following will not undergo Hofmann bromamide reaction?
CH3CONHCH3
CH3CH2CONH2
CH3CONH2
C6H5CONH2
Assertion: Acetamide on reaction with KOH and bromine gives acetic acid.
Reason: Bromine catalyses hydrolysis of acetamide.
if both assertion and reason are true and reason is the correct explanation of assertion.
if both assertion and reason are true but reason is not the correct explanation of assertion.
assertion is true but reason is false.
both assertion and reason are false.
\[\ce{CH3CH2Br ->[aq NaOH][\Delta] A ->[KMnO4/H^+][\Delta] B ->[NH3][\Delta] C ->[Br2/NaOH] D}\] ‘D’ is:
bromomethane
α-bromo sodium acetate
methanamine
acetamide
Which one of the following nitro compounds does not react with nitrous acid?
CH3 – CH2 – CH2 – NO2
(CH3)2 CH – CH2NO2
(CH3)3C NO2
\[\begin{array}{cc}
\ce{CH3 - C - CH - NO2}\\
\phantom{.}||\phantom{....}|\phantom{...}\\
\phantom{.}\ce{O}\phantom{...}\ce{CH3}\phantom{}
\end{array}\]
\[\ce{Aniline + benzoylchloride ->[NaOH] C6H5 - NH - COC6H5}\] this reaction is known as ____________.
Friedel-crafts reaction
HVZ reaction
Schotten-Baumann reaction
none of these
The product formed by the reaction an aldehyde with a primary amine ____________.
carboxylic acid
aromatic acid
schiff’s base
ketone
Which of the following reaction is not correct.
\[\ce{CH3CH2NH2 ->[HNO2] CH3CH2OH + N2}\]
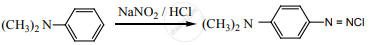
\[\ce{CH3CONH2 ->[Br2/NaOH] CH3NH2}\]
none of these
When aniline reacts with acetic anhydride the product formed is ____________.
o-aminoacetophenone
m-aminoacetophenone
p-aminoacetophenone
acetanilide
The order of basic strength for methyl substituted amines in aqueous solution is ____________.
N(CH3)3 > N(CH3)2H > N(CH3)H2 > NH3
N(CH3)H2 > N(CH3)2H > N(CH3)3 > NH3
NH3 > N(CH3)H2 > N(CH3)2H > N(CH3)3
N(CH3)2H > N(CH3)H2 > N(CH3)3 > NH3
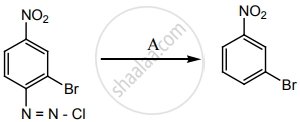
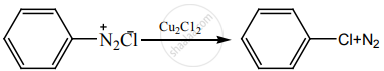
‘A’ is:
H3PO2 and H2O
H+/H2O
HgSO4/H2SO4
Cu2Cl2
\[\ce{C6H5NO2 ->[Fe/HCl] A ->[NaNO2/HCl][273 K] B ->[H2O][283 K] C}\] ‘C’ is:
C6H5 – OH
C6H5 – CH2OH
C6 H5 – CHO
C6H5NH2
Nitrobenzene on reaction with at 80-100°C forms which one of the following products?
1, 4-dinitrobenzene
2, 4, 6-trinitrobenzene
1, 2-dinitrobenzene
1, 3-dinitrobenzene
C5H13N reacts with HNO2 to give an optically active compound – The compound is ____________.
pentan-1-amine
pentan-2-amine
N, N-dimethyl propane-2-amine
N-methyl butane-2-amine
Secondary nitro alkanes react with nitrous acid to form ____________.
red solution
blue solution
green solution
yellow solution
Which of the following amines does not undergo acetylation?
t-butylamine
ethylamine
diethylamine
triethylamine
Which one of the following is most basic?
2, 4-dichloro aniline
2, 4-dimethyl aniline
2, 4-dinitro aniline
2, 4-dibromo aniline
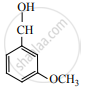
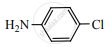
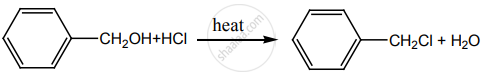
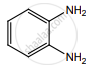
When 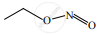 is reduced with Sn/HCl the pair of compounds formed are ____________.
is reduced with Sn/HCl the pair of compounds formed are ____________.
Ethanol, hydroxylamine hydrochloride
Ethanol, ammonium hydroxide
Ethanol, NH2OH
C3H5NH2, H2O
IUPAC name for the amine is:
\[\begin{array}{cc}
\phantom{.}\ce{CH3}\\
|\phantom{..}\\
\ce{CH3 - N - C - CH2 - CH3}\\
\phantom{.}|\phantom{.....}|\phantom{........}\\
\phantom{}\ce{CH3}\phantom{..}\ce{C2H5}\phantom{....}
\end{array}\]
3-Bimethylamino-3-methyl pentane
3(N, N-Triethyl)-3-amino pentane
3-N, N-trimethyl pentanamine
3-(N, N-dimethyl amino)-3-methyl pentane
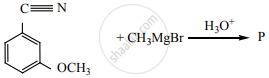
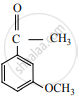
Product ‘P’ in the above reaction is:
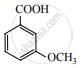
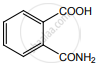
Ammonium salt of benzoic acid is heated strongly with P2O5 and the product so formed is reduced and then treated with NaNO2/HCl at low temperature. The final compound formed is ____________.
Benzene diazonium chloride
Benzyl alcohol
Phenol
Nitrosobenzene
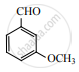
Identify X in the sequence given below
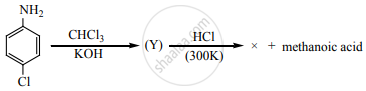
Among the following, the reaction that proceeds through an electrophilic substitution is:
The major product of the following reaction:
Short answer Questions:
Write down the possible isomers of the C4H9NO2 give their IUPAC names.
There are two isomers with the formula CH3NO2. How will you distinguish between them?
What happens when 2-Nitropropane boiled with HCl?
What happens when Nitrobenzene undergoes electrolytic reduction in a strongly acidic medium?
What happens when oxidation of tert-butylamine with KMnO4?
What happens when oxidation of acetone oxime with trifluoroperoxy acetic acid
How will you convert nitrobenzene into 1, 3, 5-trinitrobenzene?
How will you convert nitrobenzene into o and p-nitrophenol?
How will you convert nitrobenzene into m-nitro aniline?
How will you convert nitrobenzene into azoxybenzene?
How will you convert nitrobenzene into hydrozobenzene?
How will you convert nitrobenzene into N-phenylhydroxylamine?
How will you convert nitrobenzene into aniline?
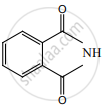
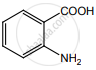
Identify compounds A, B and C in the following sequence of reaction.
\[\ce{C6H5NO2 ->[Fe/HCl] A ->[HNO2][273 K] B ->[C6H5OH] C}\]
Identify compounds A, B and C in the following sequence of reaction.
\[\ce{C6H5N2Cl ->[CuCN] A ->[H2O/H^+] B ->[NH3] C}\]
Identify compounds A, B and C in the following sequence of reaction.
\[\ce{CH3CH2I ->[NaCN] A ->[OH^-][Partial hydrolysis] B ->[NaOH + Br2] C}\]
Identify compounds A, B and C in the following sequence of reaction.
\[\ce{CH3NH2 ->[CH3Br] A ->[CH3COCl] B ->[B2H6] C}\]
Identify compounds A, B and C in the following sequence of reaction.
\[\ce{C6H5NH2 ->[(CH3CO2)O][pyridine] A ->[HNO3][H2SO4, 288 K] B ->[H2O/H^+] C}\]
Identify compounds A, B and C in the following sequence of reaction.
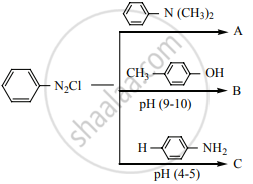
Identify compounds A, B and C in the following sequence of reaction.
\[\ce{CH3CH2NC ->[HgO] A ->[H2O] B ->[i) NaNO2/HCl][ii) H2O] C}\]
Write a short note on the following.
Hofmann’s bromide reaction
Write a short note on the following.
Ammonolysis
Write a short note on the following.
Gabriel phthalimide synthesis
Write a short note on the following.
Schotten-Baumann reaction
Write a short note on the following.
Carbylamine reaction
Write a short note on the following.
Mustard oil reaction
Write a short note on the following.
Coupling reaction
Write a short note on Diazotisation
Write a short note on the following.
Gomberg reaction
How will you distinguish between primary secondary and tertiary aliphatic amines?
Account for the following:
Aniline does not undergo Friedel-Crafts reaction.
Account for the following.
Diazonium salts of aromatic amines are more stable than those of aliphatic amines.
Account for the following.
pKb of aniline is more than that of methylamine.
Account for the following.
Gabriel phthalimide synthesis is preferred for synthesising primary amines.
Account for the following.
Ethylamine is soluble in water whereas aniline is not.
Account for the following.
Amines are more basic than amides.
Account for the following.
Although amino group is o- and p-directing in aromatic electrophilic substitution reactions, aniline on nitration gives a substantial amount of m-nitroaniline.
Arrange the following.
In increasing order of solubility in water, C6H5NH2, (C2H5)2NH, C2H5NH2
Arrange the following.
In increasing order of basic strength aniline, p-toluidine and p-nitro aniline
Arrange the following.
In increasing order of basic strength C6H5NH2, C6H5NHCH3, C6H5NH2, p-Cl-C6H4-NH2
Arrange the following.
In decreasing order of basic strength in gas phase (C2H5)NH2, (C2H5)NH, (C2H5)3N and NH3.
Arrange the following.
In increasing order of boiling point C6H5OH, (CH3)2NH, C2H5NH2.
Arrange the following.
In decreasing order of the pKb values C2H5NH2, C6H5NHCH3, (C2H)2NH and CH3NH2.
Arrange the following.
Increasing order of basic strength C6H5NH2, C6H5N(CH3)2, (C2H5)2NH and CH3NH2.
Arrange the following.
In decreasing order of basic strength
![]()
How will you prepare propan-1-amine from butane nitrile?
How will you prepare propan-1-amine from propanamide?
How will you prepare propan-1-amine from 1-nitropropane?
Identify A, B and C.
\[\ce{CH3 - NO2 ->[LiAlH4] A ->[2CH3CH2Br] B ->[H2SO4] C}\]
How will you convert diethylamine into N, N-diethyl acetamide?
How will you convert diethylamine into N-nitrosodiethylamine?
Identify A, B and C.
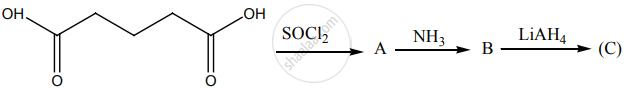
Identify A, B, C and D.
\[\ce{aniline + benzaldehyde -> A ->[Conc. HNO3][B] C + D}\]
Complete the following reaction.
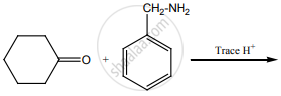
Predict A, B, C and D for the following reaction.
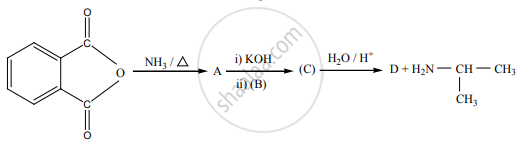
A dibromo derivative (A) on treatment with KCN followed by acid hydrolysis and heating gives a monobasic acid (B) along with the liberation of CO2. (B) on heating with liquid ammonia followed by treating with Br2/KOH gives (C) which on treating with NaNO2 and HCl at low temperature followed by oxidation gives a monobasic acid (D) having molecular mass 74. Identify A to D.
Identify A to E in the following sequence of reactions.
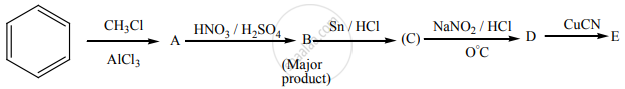
Solutions for 13: Organic Nitrogen Compounds
![Samacheer Kalvi solutions for Chemistry - Volume 1 and 2 [English] Class 12 TN Board chapter 13 - Organic Nitrogen Compounds Samacheer Kalvi solutions for Chemistry - Volume 1 and 2 [English] Class 12 TN Board chapter 13 - Organic Nitrogen Compounds - Shaalaa.com](/images/chemistry-volume-1-and-2-english-class-12-tn-board_6:5f2b1b2038084cf381bfa42c826a928c.jpg)
Samacheer Kalvi solutions for Chemistry - Volume 1 and 2 [English] Class 12 TN Board chapter 13 - Organic Nitrogen Compounds
Shaalaa.com has the Tamil Nadu Board of Secondary Education Mathematics Chemistry - Volume 1 and 2 [English] Class 12 TN Board Tamil Nadu Board of Secondary Education solutions in a manner that help students grasp basic concepts better and faster. The detailed, step-by-step solutions will help you understand the concepts better and clarify any confusion. Samacheer Kalvi solutions for Mathematics Chemistry - Volume 1 and 2 [English] Class 12 TN Board Tamil Nadu Board of Secondary Education 13 (Organic Nitrogen Compounds) include all questions with answers and detailed explanations. This will clear students' doubts about questions and improve their application skills while preparing for board exams.
Further, we at Shaalaa.com provide such solutions so students can prepare for written exams. Samacheer Kalvi textbook solutions can be a core help for self-study and provide excellent self-help guidance for students.
Concepts covered in Chemistry - Volume 1 and 2 [English] Class 12 TN Board chapter 13 Organic Nitrogen Compounds are Nitro Compounds, Classification of Amines, Introduction of Diazonium Salts, Cyanides and Isocyanides.
Using Samacheer Kalvi Chemistry - Volume 1 and 2 [English] Class 12 TN Board solutions Organic Nitrogen Compounds exercise by students is an easy way to prepare for the exams, as they involve solutions arranged chapter-wise and also page-wise. The questions involved in Samacheer Kalvi Solutions are essential questions that can be asked in the final exam. Maximum Tamil Nadu Board of Secondary Education Chemistry - Volume 1 and 2 [English] Class 12 TN Board students prefer Samacheer Kalvi Textbook Solutions to score more in exams.
Get the free view of Chapter 13, Organic Nitrogen Compounds Chemistry - Volume 1 and 2 [English] Class 12 TN Board additional questions for Mathematics Chemistry - Volume 1 and 2 [English] Class 12 TN Board Tamil Nadu Board of Secondary Education, and you can use Shaalaa.com to keep it handy for your exam preparation.