Advertisements
Advertisements
Question
Account for the following.
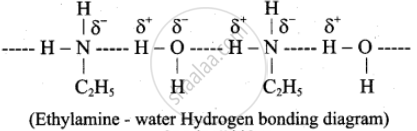
Ethylamine is soluble in water whereas aniline is not.
Solution
Ethylamine when added to water forms intermolecular H-bonds with water and therefore it is soluble in water. But aniline does not form an H-bond with water to a very large extent due to the presence of a large hydrophobic –C6H5 group. Hence, aniline is insoluble in water.
APPEARS IN
RELATED QUESTIONS
In the following
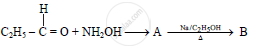
The compound ‘B’ is _______.
(A) Propan–1–amine
(B) Propan–2–amine
(C) Isopropylamine
(D) Dimethylamine
Choose the most correct option.
Carbylamine test is given by ____________.
What is the action of nitrous acid on the following compounds?
Isopropyl amine
Assertion: Acetamide on reaction with KOH and bromine gives acetic acid.
Reason: Bromine catalyses hydrolysis of acetamide.
IUPAC name for the amine is:
\[\begin{array}{cc}
\phantom{.}\ce{CH3}\\
|\phantom{..}\\
\ce{CH3 - N - C - CH2 - CH3}\\
\phantom{.}|\phantom{.....}|\phantom{........}\\
\phantom{}\ce{CH3}\phantom{..}\ce{C2H5}\phantom{....}
\end{array}\]
How will you convert nitrobenzene into m-nitro aniline?
Write a short note on the following.
Mustard oil reaction
Account for the following.
Although amino group is o- and p-directing in aromatic electrophilic substitution reactions, aniline on nitration gives a substantial amount of m-nitroaniline.
Classify the following amine as primary, secondary or tertiary:
(C2H5)2CHNH2
Account for the following:
Aniline does not undergo Friedel-Crafts reaction.
