Advertisements
Advertisements
Question
Arrange the following.
In increasing order of basic strength aniline, p-toluidine and p-nitro aniline
Solution
The electron-donating groups increase the basic strength of amines while the electron-withdrawing groups decrease the basic strength of amines. Therefore p-nitro aniline is the weakest base followed by aniline while p-toluidine, which has methyl group and therefore it is the strongest base.
Basic strength increases in the order:
P-nitro aniline < aniline < p-toluidine
APPEARS IN
RELATED QUESTIONS
Which of the following reagent can be used to convert nitrobenzene to aniline?
C5H13N reacts with HNO2 to give an optically active compound – The compound is ____________.
Secondary nitro alkanes react with nitrous acid to form ____________.
Among the following, the reaction that proceeds through an electrophilic substitution is:
What happens when oxidation of tert-butylamine with KMnO4?
What happens when oxidation of acetone oxime with trifluoroperoxy acetic acid
How will you convert nitrobenzene into 1, 3, 5-trinitrobenzene?
How will you convert nitrobenzene into N-phenylhydroxylamine?
Account for the following:
Aniline does not undergo Friedel-Crafts reaction.
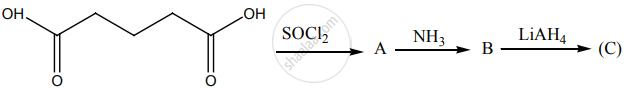
Identify A, B and C.