Advertisements
Advertisements
Question
What happens when oxidation of acetone oxime with trifluoroperoxy acetic acid
Solution
Oxidation of acetone oxime with trifluoroperoxy acetic acid gives 2-nitropropane.
\[\begin{array}{cc}
\ce{CH3 - C = N - OH ->[CF3COOH][(O)] CH3 - CH - NO2}\\
\phantom{}|\phantom{.............................}|\phantom{..}\\
\phantom{.}\ce{\underset{(Acetone oxime)}{CH3}}\phantom{..................}\ce{\underset{(2-nitropropane)}{CH3}}\phantom{}
\end{array}\]
APPEARS IN
RELATED QUESTIONS
C5H13N reacts with HNO2 to give an optically active compound – The compound is ____________.
Write down the possible isomers of the C4H9NO2 give their IUPAC names.
What happens when 2-Nitropropane boiled with HCl?
How will you convert nitrobenzene into azoxybenzene?
How will you convert nitrobenzene into hydrozobenzene?
Identify compounds A, B and C in the following sequence of reaction.
\[\ce{C6H5NO2 ->[Fe/HCl] A ->[HNO2][273 K] B ->[C6H5OH] C}\]
Identify A to E in the following sequence of reactions.
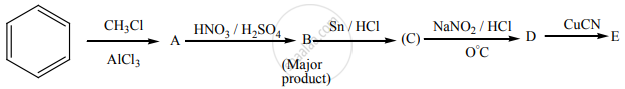
Account for the following.
Aniline does not undergo Friedel–Crafts reaction
Account for the following.
Aniline does not undergo Friedel-Crafts reaction.
Account for the following:
Aniline does not undergo Friedel – Crafts reaction.
