Advertisements
Advertisements
Question
How will you convert nitrobenzene into azoxybenzene?
Solution
Conversion of nitrobenzene into azoxybenzene:
\[\begin{array}{cc}
\ce{\underset{(nitrobenzene)}{2C6H5NO2} ->[Na3 AsO3/NaOH \Delta][(or) Glucose/NaOH \Delta] C6H5 - N = N - C6H5}\\
\phantom{........................}↓\\
\phantom{.........................}\ce{\underset{(azoxybenzene)}{O}}
\end{array}\]
APPEARS IN
RELATED QUESTIONS
Which of the following reagent can be used to convert nitrobenzene to aniline?
\[\ce{CH3CH2Br ->[aq NaOH][\Delta] A ->[KMnO4/H^+][\Delta] B ->[NH3][\Delta] C ->[Br2/NaOH] D}\] ‘D’ is:
Nitrobenzene on reaction with at 80-100°C forms which one of the following products?
When 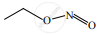 is reduced with Sn/HCl the pair of compounds formed are ____________.
is reduced with Sn/HCl the pair of compounds formed are ____________.
How will you convert nitrobenzene into o and p-nitrophenol?
How will you convert nitrobenzene into N-phenylhydroxylamine?
Arrange the following.
In increasing order of basic strength aniline, p-toluidine and p-nitro aniline
Account for the following
Aniline does not undergo Friedel–Crafts reaction
Account for the following.
Aniline does not undergo Friedel–Crafts reaction
Account for the following.
Aniline does not undergo Friedel – Crafts reaction.
