Advertisements
Advertisements
Question
Write down the possible isomers of the C4H9NO2 give their IUPAC names.
Solution
(i) \[\ce{\underset{(1-nitro butane)}{CH3 - CH2 - CH2 - CH2 - NO2}}\]
(ii) \[\begin{array}{cc}
\ce{CH3 - CH2 - CH - CH3}\\
\phantom{.....}|\\
\phantom{........}\ce{\underset{(2-nitro butane)}{NO2}}
\end{array}\]
(iii) \[\begin{array}{cc}
\ce{CH3}\phantom{......}\\
|\phantom{.........}\\
\ce{\underset{(2-methyl-1-nitro propane)}{CH3 - CH - CH2 - NO2}}
\end{array}\]
(iv) \[\begin{array}{cc}
\phantom{...}\ce{CH3}\\
|\\
\ce{CH3 - C - NO2}\\
|\\
\phantom{....}\ce{\underset{(2-methyl-2-nitro propane)}{CH3}}
\end{array}\]
(v) \[\ce{\underset{(1-nitrio butane)}{CH3 - CH2 - CH2 - CH2 - O - N = O}}\]
(vi) \[\begin{array}{cc}
\ce{CH3 - CH2 - CH - CH3}\\
\phantom{.....}|\\
\phantom{...............}\ce{\underset{(2-nitrio butane)}{O - N = O}}
\end{array}\]
(vii) \[\begin{array}{cc}
\ce{CH3}\phantom{.}\\
|\phantom{....}\\
\ce{\underset{(2-methyl-1-nitrio propane)}{CH3 - CH - CH2 - O - N = O}}
\end{array}\]
(viii) \[\begin{array}{cc}
\ce{CH3}\phantom{.....}\\
|\phantom{.......}\\
\ce{CH3 - C - O - N = O}\\
|\phantom{.......}\\
\ce{\underset{(2-methyl-2-nitrio propane)}{CH3}}\phantom{....}
\end{array}\]
APPEARS IN
RELATED QUESTIONS
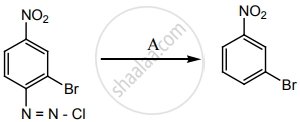
‘A’ is:
C5H13N reacts with HNO2 to give an optically active compound – The compound is ____________.
What happens when oxidation of tert-butylamine with KMnO4?
What happens when oxidation of acetone oxime with trifluoroperoxy acetic acid
Identify compounds A, B and C in the following sequence of reaction.
\[\ce{C6H5NO2 ->[Fe/HCl] A ->[HNO2][273 K] B ->[C6H5OH] C}\]
Write a short note on the following.
Hofmann’s bromide reaction
Account for the following.
Aniline does not undergo Friedel–Crafts reaction
Account for the following.
Aniline does not undergo Friedel – Crafts reaction.
Account for the following:
Aniline does not undergo Friedel - Crafts reaction.
Account for the following:
Aniline does not undergo Friedel – Crafts reaction.
