Advertisements
Advertisements
Question
Arrange the following.
In increasing order of basic strength C6H5NH2, C6H5NHCH3, C6H5NH2, p-Cl-C6H4-NH2
Solution
Chlorine atom has both – I effect and + R effect since – I effect out weights the + R effect, therefore p-chloro aniline is weak base than aniline. Alkyl groups are electron-donating groups.
As a result, the electron density on the nitrogen atom increases in the ethylamine, and thus they can donate lone pair of electrons more easily. Therefore Ethylamine is more base than aromatic amines.
Due to delocalization of the lone pair of electrons of the N-atom over the benzene ring, C6H5NH, and C6H5NHCH3 are far less basic than C2H5NH2. Further due to +I effect of the CH3 group, C6H5NHCH3 is a little more basic than C6H5NH2. Therefore increasing order basic strength is p-Cl-C6H4-NH2 < C6H5NH2 < C6H5NHCH3 < C6H5NH2.
APPEARS IN
RELATED QUESTIONS
The method by which aniline cannot be prepared is ____________.
Which one of the following nitro compounds does not react with nitrous acid?
Secondary nitro alkanes react with nitrous acid to form ____________.
There are two isomers with the formula CH3NO2. How will you distinguish between them?
How will you convert nitrobenzene into o and p-nitrophenol?
Identify compounds A, B and C in the following sequence of reaction.
\[\ce{C6H5NO2 ->[Fe/HCl] A ->[HNO2][273 K] B ->[C6H5OH] C}\]
Identify A, B and C.
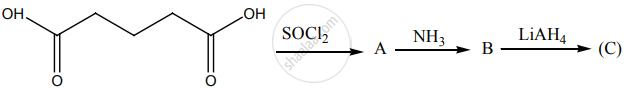
Account for the following.
Aniline does not undergo Friedel – Crafts reaction
Account for the following.
Aniline does not undergo Friedel – Crafts reaction.
Account for the following:
Aniline does not undergo Friedel - Crafts reaction.
