Advertisements
Advertisements
Question
Which one of the following nitro compounds does not react with nitrous acid?
Options
CH3 – CH2 – CH2 – NO2
(CH3)2 CH – CH2NO2
(CH3)3C NO2
\[\begin{array}{cc}
\ce{CH3 - C - CH - NO2}\\
\phantom{.}||\phantom{....}|\phantom{...}\\
\phantom{.}\ce{O}\phantom{...}\ce{CH3}\phantom{}
\end{array}\]
Solution
(CH3)3C NO2
APPEARS IN
RELATED QUESTIONS
Which of the following reagent can be used to convert nitrobenzene to aniline?
\[\ce{CH3CH2Br ->[aq NaOH][\Delta] A ->[KMnO4/H^+][\Delta] B ->[NH3][\Delta] C ->[Br2/NaOH] D}\] ‘D’ is:
Secondary nitro alkanes react with nitrous acid to form ____________.
What happens when Nitrobenzene undergoes electrolytic reduction in a strongly acidic medium?
How will you convert nitrobenzene into aniline?
Arrange the following.
In increasing order of basic strength C6H5NH2, C6H5NHCH3, C6H5NH2, p-Cl-C6H4-NH2
Identify A, B and C.
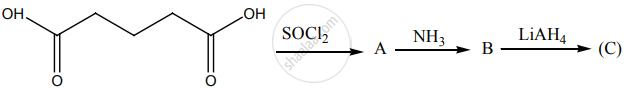
Account for the following.
Aniline does not undergo Friedel – Crafts reaction.
Account for the following:
Aniline does not undergo Friedel - Crafts reaction.
Account for the following.
Aniline does not undergo Friedel–Crafts reaction
