Advertisements
Advertisements
Question
What happens when Nitrobenzene undergoes electrolytic reduction in a strongly acidic medium?
Solution
Electrolytic reduction of nitrobenzene in weakly acidic medium gives aniline but in strongly acidic medium, it gives p-amino phenol obviously through the acid catalysed rearrangement of the initially formed phenyl hydroxylamine.

APPEARS IN
RELATED QUESTIONS
Which one of the following will not undergo Hofmann bromamide reaction?
Which one of the following nitro compounds does not react with nitrous acid?
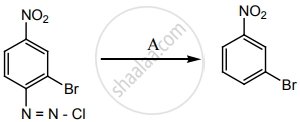
‘A’ is:
Among the following, the reaction that proceeds through an electrophilic substitution is:
There are two isomers with the formula CH3NO2. How will you distinguish between them?
How will you convert nitrobenzene into 1, 3, 5-trinitrobenzene?
How will you convert nitrobenzene into hydrozobenzene?
Write a short note on the following.
Hofmann’s bromide reaction
Account for the following.
Aniline does not undergo Friedel – Crafts reaction
Account for the following.
Aniline does not undergo Friedel–Crafts reaction
