Advertisements
Advertisements
Question
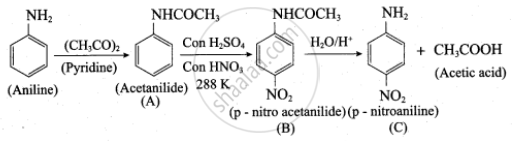
Identify compounds A, B and C in the following sequence of reaction.
\[\ce{C6H5NH2 ->[(CH3CO2)O][pyridine] A ->[HNO3][H2SO4, 288 K] B ->[H2O/H^+] C}\]
Solution
APPEARS IN
RELATED QUESTIONS
Which one of the following will not undergo Hofmann bromamide reaction?
\[\ce{CH3CH2Br ->[aq NaOH][\Delta] A ->[KMnO4/H^+][\Delta] B ->[NH3][\Delta] C ->[Br2/NaOH] D}\] ‘D’ is:
Nitrobenzene on reaction with at 80-100°C forms which one of the following products?
C5H13N reacts with HNO2 to give an optically active compound – The compound is ____________.
Among the following, the reaction that proceeds through an electrophilic substitution is:
What happens when oxidation of acetone oxime with trifluoroperoxy acetic acid
How will you convert nitrobenzene into 1, 3, 5-trinitrobenzene?
Arrange the following.
In increasing order of basic strength C6H5NH2, C6H5NHCH3, C6H5NH2, p-Cl-C6H4-NH2
Account for the following
Aniline does not undergo Friedel–Crafts reaction
Account for the following:
Aniline does not undergo Friedel - Crafts reaction.
