Advertisements
Advertisements
Question
How will you prepare propan-1-amine from 1-nitropropane?
Solution
Preparation of propan-1-amine from 1-nitropropane:
Reduction of 1-Nitropropane using H2/Ni or Fe/HCl gives propan-1-amine.
\[\ce{\underset{(1-nitropropane)}{CH3 - CH2 - CH2 - NO2} ->[Fe/HCl][6(H)] \underset{(Propan-1-amine)}{CH3 - CH2 - CH2 - NH2} + 2H2O}\]
APPEARS IN
RELATED QUESTIONS
In the following
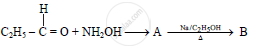
The compound ‘B’ is _______.
(A) Propan–1–amine
(B) Propan–2–amine
(C) Isopropylamine
(D) Dimethylamine
How are amines classified?
\[\ce{Aniline + benzoylchloride ->[NaOH] C6H5 - NH - COC6H5}\] this reaction is known as ____________.
Write a short note on the following.
Ammonolysis
Account for the following.
Ethylamine is soluble in water whereas aniline is not.
Account for the following.
Although amino group is o- and p-directing in aromatic electrophilic substitution reactions, aniline on nitration gives a substantial amount of m-nitroaniline.
The main product is formed by treating an alkyl or benzyl halide with excess ammonia ____________.
Write short note on the following:
Ammonolysis
Name the distinguishing test for differentiating 1° amine from 2° and 3° amine.
Write a short note on the following
Ammonolysis
