Advertisements
Advertisements
Question
Write a short note on the following.
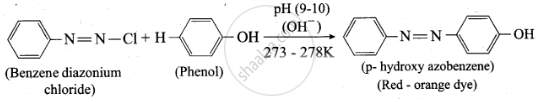
Coupling reaction
Solution
Benzene diazonium chloride reacts with electron-rich aromatic compounds like phenol, aniline to form brightly coloured azo compounds. Coupling generally occurs at the para position. If para position is occupied then coupling occurs at the ortho position. Coupling tendency is enhanced if an electron-donating group is present at the para-position to \[\ce{-\overset{+}{N2}Cl^-}\] group. This is an electrophilic substitution.
APPEARS IN
RELATED QUESTIONS
The conversion of primary aromatic amines into diazonium salts is known as ___________
Write a short note on Diazotisation
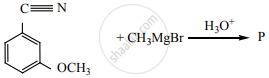
Product ‘P’ in the above reaction is:
Ammonium salt of benzoic acid is heated strongly with P2O5 and the product so formed is reduced and then treated with NaNO2/HCl at low temperature. The final compound formed is ____________.
Write a short note on the following.
Gomberg reaction
Account for the following.
Diazonium salts of aromatic amines are more stable than those of aliphatic amines.
Account for the following.
Amines are more basic than amides.
Identify A, B, C and D.
\[\ce{aniline + benzaldehyde -> A ->[Conc. HNO3][B] C + D}\]
A dibromo derivative (A) on treatment with KCN followed by acid hydrolysis and heating gives a monobasic acid (B) along with the liberation of CO2. (B) on heating with liquid ammonia followed by treating with Br2/KOH gives (C) which on treating with NaNO2 and HCl at low temperature followed by oxidation gives a monobasic acid (D) having molecular mass 74. Identify A to D.
What would be the major product of the following reaction?
\[\ce{C6H5 - CH2 - OC6H5 + HBr -> A + B}\]
