Advertisements
Advertisements
Question
Account for the following.
Diazonium salts of aromatic amines are more stable than those of aliphatic amines.
Solution
The diazonium salts of aromatic amines are more stable than those of aliphatic amines due to the dispersal of the positive charge on the benzene ring as shown below.
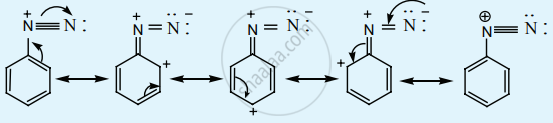
APPEARS IN
RELATED QUESTIONS
The conversion of primary aromatic amines into diazonium salts is known as ___________
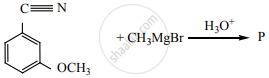
Product ‘P’ in the above reaction is:
The major product of the following reaction:
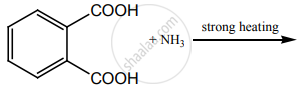
Write a short note on the following.
Coupling reaction
Write a short note on the following.
Gomberg reaction
Account for the following.
Amines are more basic than amides.
Identify A, B, C and D.
\[\ce{aniline + benzaldehyde -> A ->[Conc. HNO3][B] C + D}\]
Why is \[\ce{NH2}\] group of aniline acetylated before carrying out nitration?
What would be the major product of the following reaction?
\[\ce{C6H5 - CH2 - OC6H5 + HBr -> A + B}\]
Coupling of benzene diazonium chloride with 1-naphthol in alkaline medium will give:
