Advertisements
Advertisements
Question
Identify A, B, C and D.
\[\ce{aniline + benzaldehyde -> A ->[Conc. HNO3][B] C + D}\]
Solution
\[\ce{C6H5NH2 + C6H5CHO -> \underset{\underset{(Schiff's base)(A)}{(Benzal aniline)}}{C6H5N = CH - C6H5} ->[Conc. HNO3][\underset{(B)}{H2O}] \underset{\underset{(C)}{(Aniline)}}{C6H5NH2} + \underset{\underset{(D)}{(Benzaldehyde)}}{C6H4CHO}}\]
APPEARS IN
RELATED QUESTIONS
Write a short note on Diazotisation
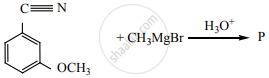
Product ‘P’ in the above reaction is:
Ammonium salt of benzoic acid is heated strongly with P2O5 and the product so formed is reduced and then treated with NaNO2/HCl at low temperature. The final compound formed is ____________.
The major product of the following reaction:
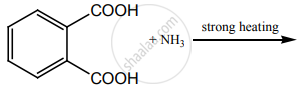
Write a short note on the following.
Coupling reaction
Write a short note on the following.
Gomberg reaction
Account for the following.
Amines are more basic than amides.
A dibromo derivative (A) on treatment with KCN followed by acid hydrolysis and heating gives a monobasic acid (B) along with the liberation of CO2. (B) on heating with liquid ammonia followed by treating with Br2/KOH gives (C) which on treating with NaNO2 and HCl at low temperature followed by oxidation gives a monobasic acid (D) having molecular mass 74. Identify A to D.
Why is \[\ce{NH2}\] group of aniline acetylated before carrying out nitration?
What would be the major product of the following reaction?
\[\ce{C6H5 - CH2 - OC6H5 + HBr -> A + B}\]
