Advertisements
Advertisements
Question
A dibromo derivative (A) on treatment with KCN followed by acid hydrolysis and heating gives a monobasic acid (B) along with the liberation of CO2. (B) on heating with liquid ammonia followed by treating with Br2/KOH gives (C) which on treating with NaNO2 and HCl at low temperature followed by oxidation gives a monobasic acid (D) having molecular mass 74. Identify A to D.
Solution
(i) \[\begin{array}{cc}
\ce{CH3 - CH2 - CH - CH2 ->[KCN] CH3 - CH2 - CH - CH2 ->[(i) H^+/H2O][(ii) Heat] CO2 + \underset{\underset{(B)}{(Butanoic acid)}}{CH3 - CH2 - CH2} - COOH}\\
\phantom{....}|\phantom{.....}|\phantom{.........................}|\phantom{......}|\phantom{......................................}\\
\phantom{}\ce{\underset{\underset{(A)}{(1, 2-dibromobutane)}}{Br\phantom{...}Br}}\phantom{.................}\ce{\underset{(1, 2-dicyanobutane)}{CN\phantom{...}CN}}\phantom{..................................}
\end{array}\]
(ii) \[\ce{\underset{\underset{(B)}{(Butanoic acid)}}{CH3 - CH2 - CH2 - COOH} ->[liq. NH3][\Delta] \underset{(Butanamide)}{CH3 - CH2 - CH2 - CONH2} ->[Br2/KOH] \underset{\underset{(C)}{(1-Aminopropane)}}{CH3 - CH2 - CH2 - NH2}}\]
(iii) \[\ce{\underset{\underset{(C)}{1-Aminopropane}}{CH3 - CH2 - CH2 - NH2} ->[NaNO2/HCl][Low temperature] \underset{(1-propanol)}{CH3 - CH2 - CH2 - OH} ->[K2Cr2O7/H^+][2(O)] \underset{\underset{(D)}{(Propanoic acid)}}{CH3 - CH2 - COOH}}\]
(iv) Molecular mass of propanoic acid is 74.
| A |
\[\begin{array}{cc} |
1, 2-dibromo butane |
| B | \[\begin{array}{cc} \ce{CH3 - CH2 - CH - CH2 - CN}\\ |\phantom{.}\\ \phantom{.}\ce{CN} \end{array}\] |
1, 2-dicyano butane |
| C | \[\ce{CH3 - CH2 - CH2 - NH2}\] | 1-amino propane |
| D | \[\ce{CH3 - CH2 - COOH}\] | propanoic acid |
APPEARS IN
RELATED QUESTIONS
The conversion of primary aromatic amines into diazonium salts is known as ___________
Write a short note on diazotisation.
Ammonium salt of benzoic acid is heated strongly with P2O5 and the product so formed is reduced and then treated with NaNO2/HCl at low temperature. The final compound formed is ____________.
Identify X in the sequence given below
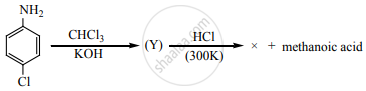
The major product of the following reaction:
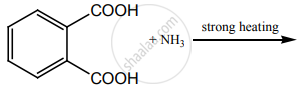
Write a short note on the following.
Gomberg reaction
Account for the following.
Diazonium salts of aromatic amines are more stable than those of aliphatic amines.
Identify A, B, C and D.
\[\ce{aniline + benzaldehyde -> A ->[Conc. HNO3][B] C + D}\]
Why is \[\ce{NH2}\] group of aniline acetylated before carrying out nitration?
What would be the major product of the following reaction?
\[\ce{C6H5 - CH2 - OC6H5 + HBr -> A + B}\]
