Advertisements
Advertisements
Question
Arrange the following.
In increasing order of boiling point C6H5OH, (CH3)2NH, C2H5NH2.
Solution
Since the electronegativity of O is higher than that of N, therefore, alcohols form stronger H-O bonds than amines. In other words, the boiling points of alcohols are higher than those of amines of comparable molecular masses. Therefore the boiling point of C6H5OH (46) is higher than those of (CH3)2NH (45) and C2H5NH2 (45).
Further, the extent of H-bonding depends upon the number of H-atoms on the N-atom. Therefore 1° amine with two H-atoms on the N-atom has higher boiling points than 2° amines having only one H-atom. Therefore the boiling point of C2H5NH2 is higher than that of(CH3)2NH.
The increasing order of boiling point is, (CH3)2NH < C2H5NH2 < C6H5OH.
APPEARS IN
RELATED QUESTIONS
In the following
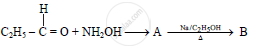
The compound ‘B’ is _______.
(A) Propan–1–amine
(B) Propan–2–amine
(C) Isopropylamine
(D) Dimethylamine
Give reasons for the following:
CH3NH2 is more basis than C6H5NH2.
Choose the most correct option.
Carbylamine test is given by ____________.
\[\ce{C6H5NO2 ->[Fe/HCl] A ->[NaNO2/HCl][273 K] B ->[H2O][283 K] C}\] ‘C’ is:
Account for the following.
pKb of aniline is more than that of methylamine.
Arrange the following.
Increasing order of basic strength C6H5NH2, C6H5N(CH3)2, (C2H5)2NH and CH3NH2.
Arrange the following.
In decreasing order of basic strength
![]()
Classify the following amine as primary, secondary or tertiary:
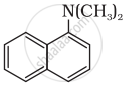
Write short note on the following:
Ammonolysis
Write a short note on the following.
Ammonolysis
