Advertisements
Advertisements
प्रश्न
Arrange the following.
In decreasing order of basic strength
![]()
उत्तर
Aliphatic amines are more basic than aromatic amines. Therefore CH3CH2NH2 and CH3NH2 are more basic. Among the ethylamine and methylamine. ethylamine was experienced more +I effect than methylamine and hence ethylamine is more basic than methylamine.
Nitro group has a powerful electron-withdrawing group and they have both – R effect as well as – I effect. As a result, all the nitro anilines are weaker bases than aniline. In P-nitro aniline
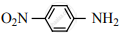
both – R effect and – I effect of the NO2 group decrease the basicity. Therefore decreasing order of basic strength is,
![]()
Ethylamine > Methylamine > Aniline > p-nitro aniline
APPEARS IN
संबंधित प्रश्न
Write the number of moles of ethanoyl chloride required for complete acylation of N, N-dimethylaniline.
Assertion: Acetamide on reaction with KOH and bromine gives acetic acid.
Reason: Bromine catalyses hydrolysis of acetamide.
\[\ce{Aniline + benzoylchloride ->[NaOH] C6H5 - NH - COC6H5}\] this reaction is known as ____________.
Write a short note on the following.
Ammonolysis
Write a short note on the following.
Schotten-Baumann reaction
Write a short note on the following.
Mustard oil reaction
Identify A, B and C.
\[\ce{CH3 - NO2 ->[LiAlH4] A ->[2CH3CH2Br] B ->[H2SO4] C}\]
The following amine can be classified as:
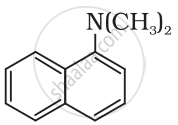
Classify the following amine as primary, secondary or tertiary:
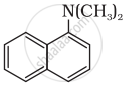
Which among the following is the strongest Bronsted base?
