Advertisements
Advertisements
प्रश्न
Choose the most correct option.
Which type of amine does produce N2 when treated with HNO2?
विकल्प
Primary amine
Secondary amine
Tertiary amine
Both primary and secondary amines
उत्तर
Primary amine
APPEARS IN
संबंधित प्रश्न
Choose the most correct option.
The hybridisation of nitrogen in primary amine is ____________.
Isobutylamine is an example of ______.
Choose the most correct option.
Carbylamine test is given by ____________.
Choose the most correct option.
Identify ‘B’ in the following reactions
\[\ce{CH3 - C ≡ N ->[Na/C2H5OH] A ->[NaNO2/dilHCl]B}\]
What are amines?
How are amines classified?
Assertion: Acetamide on reaction with KOH and bromine gives acetic acid.
Reason: Bromine catalyses hydrolysis of acetamide.
The product formed by the reaction an aldehyde with a primary amine ____________.
\[\ce{C6H5NO2 ->[Fe/HCl] A ->[NaNO2/HCl][273 K] B ->[H2O][283 K] C}\] ‘C’ is:
Which one of the following is most basic?
How will you convert nitrobenzene into m-nitro aniline?
Write a short note on the following.
Gabriel phthalimide synthesis
Write a short note on the following.
Schotten-Baumann reaction
Write a short note on the following.
Carbylamine reaction
How will you distinguish between primary secondary and tertiary aliphatic amines?
Account for the following.
pKb of aniline is more than that of methylamine.
Account for the following.
Gabriel phthalimide synthesis is preferred for synthesising primary amines.
Account for the following.
Ethylamine is soluble in water whereas aniline is not.
Arrange the following:
In increasing order of solubility in water:
C6H5NH2, (C2H5)2NH, C2H5NH2
Arrange the following.
In decreasing order of basic strength in gas phase (C2H5)NH2, (C2H5)NH, (C2H5)3N and NH3.
Arrange the following.
Increasing order of basic strength C6H5NH2, C6H5N(CH3)2, (C2H5)2NH and CH3NH2.
Arrange the following.
In decreasing order of basic strength
![]()
Identify A, B and C.
\[\ce{CH3 - NO2 ->[LiAlH4] A ->[2CH3CH2Br] B ->[H2SO4] C}\]
How will you convert diethylamine into N, N-diethyl acetamide?
How will you convert diethylamine into N-nitrosodiethylamine?
(C2H5)2CHNH2. The following amine can be classified as:
The following amine can be classified as (C2H5)2NH:
Arrange the following in the increasing order of their basic strength:
C2H5NH2, C6H5NH2, (C2H5)2NH
Complete the following reaction.
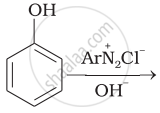
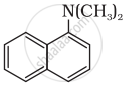
The reaction of NHO2 with 'A' gives quartering ammonium salt. A is which of the following?
\[\begin{array}{cc}
\ce{O}\phantom{.........}\\
||\phantom{.........}\\
\ce{H - \underset{(A)}{C} - NH - CH3}
\end{array}\]
and
\[\begin{array}{cc}
\ce{O}\\
||\\
\ce{CH3 - \underset{(B)}{C} - NH2}
\end{array}\]
are which type of isomers?
Classify the following amine as primary, secondary or tertiary:
Arrange the increasing order of solubility in water.
\[\ce{C2H5Cl, C2H5NH2, C2H5OH}\]
Write short note on the following:
Ammonolysis
Write a short note on the following.
Ammonolysis
Write short note on the following.
Ammonolysis
Write a short note on the following
Ammonolysis
Account for the following:
Aniline does not undergo Friedel-Crafts reaction.
Write short note on the following.
Ammonolysis
Write short note on Ammonolysis.
Write short note on the following:
Ammonolysis
Write a short note on the following.
Ammonolysis
Write a short note on the following.
Ammonolysis
Write a short note on the following.
Ammonolysis
