Advertisements
Advertisements
प्रश्न
Account for the following.
pKb of aniline is more than that of methylamine.
उत्तर
In aniline, the lone pair of electrons on the N-atom is delocalized over the benzene ring. As a result electron density on the nitrogen decreases. In contrast in CH3NH2, the +I effect of CH3 increases the electron density on the N-atom. Therefore, aniline is a weaker base than methylamine and hence its pKb value is more than that of methyl amine.
APPEARS IN
संबंधित प्रश्न
Choose the most correct option.
Which type of amine does produce N2 when treated with HNO2?
Choose the most correct option.
Identify ‘B’ in the following reactions
\[\ce{CH3 - C ≡ N ->[Na/C2H5OH] A ->[NaNO2/dilHCl]B}\]
Assertion: Acetamide on reaction with KOH and bromine gives acetic acid.
Reason: Bromine catalyses hydrolysis of acetamide.
\[\ce{C6H5NO2 ->[Fe/HCl] A ->[NaNO2/HCl][273 K] B ->[H2O][283 K] C}\] ‘C’ is:
Account for the following.
Gabriel phthalimide synthesis is preferred for synthesising primary amines.
How will you convert diethylamine into N, N-diethyl acetamide?
The following amine can be classified as (C2H5)2NH:
Classify the following amine as primary, secondary or tertiary:
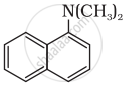
Among the following, which is the strongest base?
Write short note on the following.
Ammonolysis
