Advertisements
Advertisements
Question
What are amines?
Solution
Amines are nitrogen-containing organic compounds having basic character.
APPEARS IN
RELATED QUESTIONS
Choose the most correct option.
The hybridisation of nitrogen in primary amine is ____________.
Isobutylamine is an example of ______.
Choose the most correct option.
Which type of amine does produce N2 when treated with HNO2?
How are amines classified?
Write the number of moles of ethanoyl chloride required for complete acylation of N, N-dimethylaniline.
What is the action of nitrous acid on the following compounds?
Isopropyl amine
A tertiary amine is an organic compound ____________.
Which of the following amines is most basic in nature in aqueous phase?
Assertion: Acetamide on reaction with KOH and bromine gives acetic acid.
Reason: Bromine catalyses hydrolysis of acetamide.
\[\ce{Aniline + benzoylchloride ->[NaOH] C6H5 - NH - COC6H5}\] this reaction is known as ____________.
When aniline reacts with acetic anhydride the product formed is ____________.
The order of basic strength for methyl substituted amines in aqueous solution is ____________.
Which of the following amines does not undergo acetylation?
Which one of the following is most basic?
How will you convert nitrobenzene into m-nitro aniline?
Write a short note on the following.
Ammonolysis
Write a short note on the following.
Gabriel phthalimide synthesis
Write a short note on the following.
Schotten-Baumann reaction
Account for the following.
Gabriel phthalimide synthesis is preferred for synthesising primary amines.
Account for the following.
Ethylamine is soluble in water whereas aniline is not.
Account for the following.
Although amino group is o- and p-directing in aromatic electrophilic substitution reactions, aniline on nitration gives a substantial amount of m-nitroaniline.
Arrange the following.
In increasing order of solubility in water, C6H5NH2, (C2H5)2NH, C2H5NH2
Arrange the following.
In decreasing order of the pKb values C2H5NH2, C6H5NHCH3, (C2H)2NH and CH3NH2.
Arrange the following.
Increasing order of basic strength C6H5NH2, C6H5N(CH3)2, (C2H5)2NH and CH3NH2.
How will you prepare propan-1-amine from butane nitrile?
How will you prepare propan-1-amine from propanamide?
How will you prepare propan-1-amine from 1-nitropropane?
How will you convert diethylamine into N, N-diethyl acetamide?
The following amine can be classified as (C2H5)2NH:
The following amine can be classified as:
\[\begin{array}{cc}
\ce{O}\phantom{.........}\\
||\phantom{.........}\\
\ce{H - \underset{(A)}{C} - NH - CH3}
\end{array}\]
and
\[\begin{array}{cc}
\ce{O}\\
||\\
\ce{CH3 - \underset{(B)}{C} - NH2}
\end{array}\]
are which type of isomers?
Classify the following amine as primary, secondary or tertiary:

Classify the following amine as primary, secondary or tertiary:
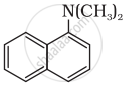
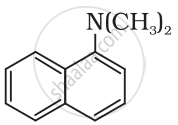
Classify the following amine as primary, secondary or tertiary:
(C2H5)2CHNH2
Which among the following is the strongest Bronsted base?
Among the following, which is the strongest base?
Arrange the increasing order of solubility in water.
\[\ce{C2H5Cl, C2H5NH2, C2H5OH}\]
Name the distinguishing test for differentiating 1° amine from 2° and 3° amine.
Write a short note on the following.
Ammonolysis
Write short note on the following.
Ammonolysis
Write short note on Ammonolysis.
Write a short note on the following.
Ammonolysis
Write a short note on the following.
Ammonolysis
