Advertisements
Advertisements
Question
Answer in brief.
Give a simple chemical test to distinguish between ethanol and ethyl bromide.
Solution
i. Ethanol on reaction with a very strong base like alkali metal Na or K gives sodium or potassium ethoxide with the liberation of hydrogen gas.
\[\ce{2CH3CH2 - OH + 2Na -> 2CH3CH2O^-Na+ + H2_{(g)}↑}\]
ii. Ethyl bromide on reaction with sodium metal does not liberate hydrogen gas.
\[\ce{2CH3CH2Br + 2Na -> CH3CH2 - CH2CH3 + 2NaBr}\]
APPEARS IN
RELATED QUESTIONS
Choose the correct option.
Which is the most resistant alcohol towards oxidation reaction among the following?
Answer in one sentence/ word.
Write the IUPAC name of alcohol having molecular formula C4H10O which is resistant towards oxidation.
Reaction between Grignard reagent and aldehyde other than formaldehyde leads to formation of _______________
Write IUPAC name of crotonyl alcohol.
Write the name of major product when anisole reacts HI at 398 K
Write the reactions involved in the preparation of phenol from benzene sulfonic acid.
What is the action of following on phenol at low temperature?
- dil. HNO3
- conc. H2SO4
- Br2/CS2
How will you bring about the following conversions?
isopropyl alcohol to acetone
What is the action of conc. H2SO4 on carbolic acid at 373 K.
Phenol reacts with concentrated nitric acid in the presence of cone. H2SO4 to form ____________.
In the Lucas test for alcohols, the appearance of turbidity is due to the formation of ____________.
In phenols, −OH group is attached to ___________ hybridised carbon.
α-butylene when subjected to hydroboration oxidation reaction, yields ______.
Phenol is obtained from cumene ____________.
Phenol is ____________.
Which of following elements does not react with hot concentrated sulphuric acid?
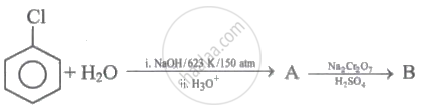
Identify 'A' and 'B' in the following series of reactions.
The most resistant alcohol towards oxidation reaction is:
Which of the following reagents is used to convert phenol to benzene?
Identify the product B in following conversion.
\[\ce{Chlorobenzene + H2O ->[Cu, 673 K][Pressure] A ->[conc. H2SO4][373 K] B}\]
Identify the compound having highest boiling point from the following?
The compound which reacts fastest with Lucas reagent at room temperature is ______.
Identify the compound amongst the following of which 0.1 M aqueous solution has highest boiling point.
The acid, which contains both -OH and -COOR groups is ______.
Arrange O - H, C - H and N - H bonds in increasing order of their bond polarity.
Write the product when 1°, 2° and 3° alcohol vapours are passed over hot copper.
Convert the following :
cumene to phenol.
