Advertisements
Advertisements
Question
What is the action of following on phenol at low temperature?
- dil. HNO3
- conc. H2SO4
- Br2/CS2
Solution
- Dilute HNO3: Phenol reacts with dilute nitric acid at low temperature to give a mixture of ortho- and para-nitrophenol.
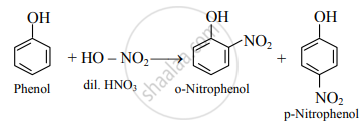
- Conc. H2SO4: At room temperature (298 K), phenol reacts with concentrated sulphuric acid to form o-phenolsulphonic acid.
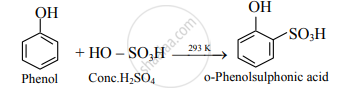
- Br2 in CS2: When a reaction is carried out in a solvent of lower polarity like CS2 a mixture of ortho- and para-bromophenol is formed.

RELATED QUESTIONS
Choose the correct option.
Which is the most resistant alcohol towards oxidation reaction among the following?
Answer in one sentence/ word.
Write the name of the electrophile used in Kolbe’s Reaction.
Answer in brief.
Explain why p-nitrophenol is a stronger acid than phenol.
Oxidation of ethyl alcohol using K2Cr2O7/dil H2SO4 leads to formation of _______________
Reaction between hot conc. HI and anisole gives ______________
Arrange the following in decreasing order of acid strength.
CH3OH, CH3–CH2–OH, CH3–CH(OH)–CH3, (CH3)3–C–OH
With the help of chemical equations show what happens when cumene hydroperoxide is treated with dil. acid.
How will you bring about the following conversions?
acetone to 2-methylpropan-2-ol
What is the action of conc. H2SO4 on carbolic acid at 373 K.
Which alcohol is difficult to oxidise?
Which of the following compounds does not react with bromine in alkaline medium?
In phenols, −OH group is attached to ___________ hybridised carbon.
Bromination of phenol, will NOT give:
Phenol is ____________.
Identify 'A' and 'B' in the following series of reactions.
The product 'C' in the following reaction is:
\[\ce{CH3CH2Br ->[alc. KCN] {'A'} ->[H3O^+][\Delta] {'B'} ->[i. LiAlH4][ii. H3O^+] {'C'}}\]
Phenoxide ion is more stable than phenol due to the ____________.
The number of moles of hydrogen gas formed when 2 moles of 2-methylpropan-2-ol reacts with aluminium is ____________.
Which among the following is allylic secondary alcohol?
A reaction of phenol with chloroform in presence of sodium hydroxide to form salicylaldehyde is known as ____________.
Identify the compound amongst the following of which 0.1 M aqueous solution has highest boiling point.

Product (B) in this reaction is:
Amongst the following alcohols which would react fastest with cone. HCl and ZnCl2?
The product C in the following reaction is

Arrange the following compounds in an increasing order of their solubility in water:
Write the chemical reaction when hot copper is treated with Vapours of 1° (primary) alcohol.
Write the chemical reaction when hot copper is treated with Vapours of 2° (secondary) alcohol.
Write chemical reaction when hot copper is treated with Vapours of 3° (tertiary) alcohol.
