Advertisements
Advertisements
Question
An ether (A), C5H12O, when heated with excess of hot HI produce two alkyl halides which on hydrolysis form compound (B) and (C), oxidation of (B) gave and acid (D), whereas oxidation of (C) gave a ketone (E). Deduce the structural formula of (A), (B), (C), (D), and (E).
Solution
i. The ether (A) with molecular formula C5H12O is
\[\begin{array}{cc}\ce{H3C - CH2 - O - HC -CH3}\\\phantom{............}|\phantom{}\\ \phantom{............}\ce{CH3\phantom{}}\end{array}\]
ii. Reacts with hot HI to produce two alkyl halides as follows:
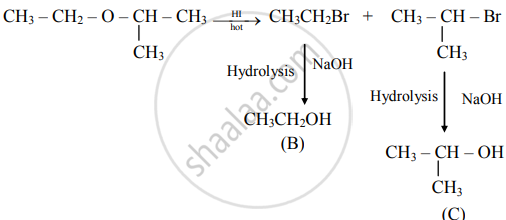
iii. Oxidation of (B) gives acid
\[\ce{CH3 - CH2 - OH ->[{[O]}] \underset{\text{(D)}}{CH3COOH}}\]
iv. Oxidation of (C) gives ketone
\[\begin{array}{cc}
\ce{CH3 - CH - OH ->[{[O]}] CH3 - C = O}\\
\phantom{...}|\phantom{....................}|\\
\phantom{....}\ce{CH3}\phantom{...........}\ce{(E)}\phantom{...}\ce{CH3}
\end{array}\]
Hence, structural formulae of compounds (A) to (E) are
(A)
\[\begin{array}{cc}\ce{CH3 - CH2 - O - CH - CH3} \\\phantom{.......................}|\phantom{...........}\\ \phantom{.......................}\ce{CH3\phantom{...........}}\end{array}\]
(2-Ethoxypropane)
(B)
CH3CH2–OH (Ethanol)
(c)
\[\begin{array}{cc}\ce{CH3 - CH - CH3}\\|\phantom{}\\ \ce{OH\phantom{}}\end{array}\]
(Propan-2-ol)
(D)
CH3COOH (Ethanoic acid)
(E)
\[\begin{array}{cc}
\ce{CH3 - CH - CH3}\\
\phantom{...}||\phantom{.....}\\
\phantom{...}
\ce{O}\phantom{.....}\end{array}\]
(Propanone)
APPEARS IN
RELATED QUESTIONS
Choose the correct option.
Which is the most resistant alcohol towards oxidation reaction among the following?
Answer in brief.
Give the reagents and conditions necessary to prepare phenol from Benzene sulfonic acid.
Answer in brief.
Give a simple chemical test to distinguish between ethanol and ethyl bromide.
Reaction between Grignard reagent and aldehyde other than formaldehyde leads to formation of _______________
Reaction between hot conc. HI and anisole gives ______________
Write the name of major product when anisole reacts HI at 398 K
What is the action of following reagents on pent-3-enal?
- H2/Ni
- LiAlH4/H3O+
Write the reactions involved in the preparation of phenol from benzene sulfonic acid.
Number of oxygen atoms present in salicylaldehyde are ______.
Which of the following compounds is obtained, when phenol react with bromine water?
α-butylene when subjected to hydroboration oxidation reaction, yields ______.
Phenol is obtained from cumene ____________.
Sodium metal with ethyl alcohol gives __________ gas.
Which of following elements does not react with hot concentrated sulphuric acid?
Cumene is used in the commercial method for the manufacture of ____________.
What is the product of the following reaction?
\[\ce{CH3 - CH2 - CH2 - OH ->[conc. H2SO4][\Delta]}\]
Identify 'A' and 'B' in the following series of reactions.
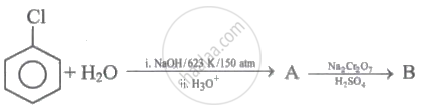
The product 'C' in the following reaction is:
\[\ce{CH3CH2Br ->[alc. KCN] {'A'} ->[H3O^+][\Delta] {'B'} ->[i. LiAlH4][ii. H3O^+] {'C'}}\]
Phenoxide ion is more stable than phenol due to the ____________.
Sodium benzene sulphonate reacts with NaOH and then on acidic hydrolysis, it gives __________.
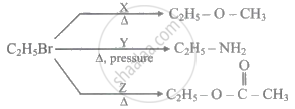
Identify reagents X, Y and Z.
The number of moles of hydrogen gas formed when 2 moles of 2-methylpropan-2-ol reacts with aluminium is ____________.
What is INCORRECT about the product written in the below given reaction?
\[\ce{R - CH2 - OH ->[PCC] R - COOH}\]
Which of the following conversion explains the acidic nature of alcohols?
Which following reagent is used to obtain alkene from alcohol?
Which among the following is not the method of preparation of phenol?
The major product obtained in the following reaction is

Arrange O - H, C - H and N - H bonds in increasing order of their bond polarity.
Write the chemical reaction when hot copper is treated with Vapours of 1° (primary) alcohol.
