Advertisements
Online Mock Tests
Chapters
2: Solutions
3: Ionic Equilibria
4: Chemical Thermodynamics
5: Electrochemistry
6: Chemical Kinetics
7: Elements of Groups 16, 17 and 18
8: Transition and Inner transition Elements
9: Coordination Compounds
10: Halogen Derivatives
11: Alcohols, Phenols and Ethers
▶ 12: Aldehydes, Ketones and Carboxylic acids
13: Amines
14: Biomolecules
15: Introduction to Polymer Chemistry
16: Green Chemistry and Nanochemistry
![Balbharati solutions for Chemistry [English] 12 Standard HSC chapter 12 - Aldehydes, Ketones and Carboxylic acids Balbharati solutions for Chemistry [English] 12 Standard HSC chapter 12 - Aldehydes, Ketones and Carboxylic acids - Shaalaa.com](/images/chemistry-english-12-standard-hsc_6:74400b801d4c44ef8e058ff9d9dfe964.jpg)
Advertisements
Solutions for Chapter 12: Aldehydes, Ketones and Carboxylic acids
Below listed, you can find solutions for Chapter 12 of Maharashtra State Board Balbharati for Chemistry [English] 12 Standard HSC.
Balbharati solutions for Chemistry [English] 12 Standard HSC 12 Aldehydes, Ketones and Carboxylic acids Exercises [Pages 280 - 281]
Choose the most correct option.
In the following resonating structures A and B, the number of unshared electrons in valence shell present on oxygen respectively are ______.
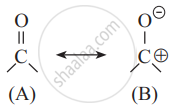
2, 4
2, 6
4, 6
6, 4
Choose the most correct option.
In the Wolff-Kishner reduction, alkyl aryl ketones are reduced to alkyl benzenes. During this change, ketones are first converted into ______.
acids
alcohols
hydrazones
alkenes
Choose the most correct option.
Aldol condensation is
electrophilic substitution reaction
nucleophilic substitution reaction
elimination reaction
addition - elimination reaction
Choose the most correct option.
Which one of the following has the lowest acidity?
Choose the most correct option.
Diborane reduces
ester group
nitro group
halo group
acid group
Choose the most correct option.
Benzaldehyde does NOT show positive test with ______.
Schiff reagent
Tollens' reagent
Sodium bisulphite solution
Fehling solution
Answer the following in one sentence.
What are aromatic ketones?
Answer the following in one sentence.
Is phenyl acetic acid an aromatic carboxylic acid?
Answer the following in one sentence.
Write reaction showing conversion of ethanenitrile into ethanol.
Answer the following in one sentence.
Predict the product of the following reaction:
\[\ce{CH3 - CH2 - COOCH3->[i) AIH(i-Bu)_2][ii) H3O+]}\]?
Answer the following in one sentence.
Name the product obtained by reacting toluene with carbon monoxide and hydrogen chloride in presence of anhydrous aluminium chloride.
Answer the following in one sentence.
Write reaction showing conversion of benzonitrile into benzoic acid.
Answer the following in one sentence.
Name the product obtained by the oxidation of 1,2,3,4-tetrahydronaphthalene with acidified potassium permanganate.
Answer the following in one sentence.
What is formalin?
Answer the following in one sentence.
Arrange the following compounds in the increasing order of their boiling points:
Formaldehyde, ethane, methyl alcohol.
Answer the following in one sentence.
Acetic acid is prepared from methyl magnesium bromide and dry ice in presence of dry ether. Name the compound which serves not only as reagent but also as a cooling agent in the reaction.
Answer in brief.
Observe the following equation of reaction of Tollens' reagent with aldehyde. How do we know that a redox reaction has taken place? Explain.
\[\ce{R-CHO + 2Ag(NH_{3})^{+}_{2} + OH^{-}->[\triangle] R-CO{O}^{-} + 2Ag↓ + 4NH3 + 2H2O}\]
Answer in brief.
Formic acid is stronger than acetic acid. Explain.
Answer in brief.
What is the action of hydrazine on cyclopentanone in presence of KOH in ethylene glycol?
Answer in brief.
Write reaction showing conversion of Acetaldehyde into acetaldehyde dimethyl acetal.
Aldehydes are more reactive towards nucleophilic addition reactions than ketones. Explain.
Answer in brief.
Write reaction showing the action of the following reagent on propanenitrile:
Dilute NaOH
Answer in brief.
Write reaction showing the action of the following reagent on propanenitrile:
Dilute HCl
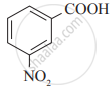
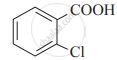
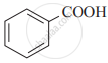
Arrange the following carboxylic acids with increasing order of their acidic strength and justify your answer.
1) ![]()
2) ![]()
3) ![]()
Describe the following:
Cannizzaro reaction
Write a note on Stephen reaction.
What is the action of the following reagent on toluene?
Alkaline KMnO4, dil. HCl and heat
What is the action of the following reagent on toluene?
CrO2Cl2 in CS2
What is the action of the following reagent on toluene?
Acetyl chloride in presence of anhydrous AlCl3.
Write the IUPAC name of the following structure:
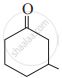
Write the IUPAC name of the following structure:
\[\begin{array}{cc}\ce{COOH}\\|\phantom{......}\\\ce{COOH}\end{array}\]
Write reaction showing conversion of p-bromoisopropylbenzene into p-isopropylbenzoic acid (3 steps).
Write reaction showing aldol condensation of cyclohexanone.
Solutions for 12: Aldehydes, Ketones and Carboxylic acids
![Balbharati solutions for Chemistry [English] 12 Standard HSC chapter 12 - Aldehydes, Ketones and Carboxylic acids Balbharati solutions for Chemistry [English] 12 Standard HSC chapter 12 - Aldehydes, Ketones and Carboxylic acids - Shaalaa.com](/images/chemistry-english-12-standard-hsc_6:74400b801d4c44ef8e058ff9d9dfe964.jpg)
Balbharati solutions for Chemistry [English] 12 Standard HSC chapter 12 - Aldehydes, Ketones and Carboxylic acids
Shaalaa.com has the Maharashtra State Board Mathematics Chemistry [English] 12 Standard HSC Maharashtra State Board solutions in a manner that help students grasp basic concepts better and faster. The detailed, step-by-step solutions will help you understand the concepts better and clarify any confusion. Balbharati solutions for Mathematics Chemistry [English] 12 Standard HSC Maharashtra State Board 12 (Aldehydes, Ketones and Carboxylic acids) include all questions with answers and detailed explanations. This will clear students' doubts about questions and improve their application skills while preparing for board exams.
Further, we at Shaalaa.com provide such solutions so students can prepare for written exams. Balbharati textbook solutions can be a core help for self-study and provide excellent self-help guidance for students.
Concepts covered in Chemistry [English] 12 Standard HSC chapter 12 Aldehydes, Ketones and Carboxylic acids are Introduction of Aldehydes, Ketones and Carboxylic Acids, Classification of Aldehydes, Ketones and Carboxylic Acids, Nomenclature of Aldehydes, Ketones and Carboxylic Acids, Preparation of Aldehydes and Ketones, Preparation of Carboxylic Acids, Physical Properties, Polarity of Carbonyl Group, Chemical Properties of Aldehydes and Ketones, Chemical Properties of Carboxylic Acids, Chemical Reactions of Aldehydes and Ketones - Reactions Due to α-hydrogen.
Using Balbharati Chemistry [English] 12 Standard HSC solutions Aldehydes, Ketones and Carboxylic acids exercise by students is an easy way to prepare for the exams, as they involve solutions arranged chapter-wise and also page-wise. The questions involved in Balbharati Solutions are essential questions that can be asked in the final exam. Maximum Maharashtra State Board Chemistry [English] 12 Standard HSC students prefer Balbharati Textbook Solutions to score more in exams.
Get the free view of Chapter 12, Aldehydes, Ketones and Carboxylic acids Chemistry [English] 12 Standard HSC additional questions for Mathematics Chemistry [English] 12 Standard HSC Maharashtra State Board, and you can use Shaalaa.com to keep it handy for your exam preparation.