Advertisements
Advertisements
Question
Answer in brief.
Observe the following equation of reaction of Tollens' reagent with aldehyde. How do we know that a redox reaction has taken place? Explain.
\[\ce{R-CHO + 2Ag(NH_{3})^{+}_{2} + OH^{-}->[\triangle] R-CO{O}^{-} + 2Ag↓ + 4NH3 + 2H2O}\]
Solution
When an aldehyde is boiled with Tollens' reagent, it gets oxidized to carboxylate ion and Ag+ ion is reduced to silver (Ag). The oxidation number of carbon in aldehyde increases while the oxidation number of Ag decreases. Hence, this is a redox reaction.
APPEARS IN
RELATED QUESTIONS
Write reaction showing aldol condensation of cyclohexanone.
Write reactions for the action of the following reagents on p-chlorobenzaldehyde.
Phenyl hydrazine
ln which of the following reactions, both oxidised and reduced forms of the same compound are obtained?
Identify B in the following reaction,
\[\ce{Acetaldoxime ->[Na][alcohol] A ->[NaNO2][HCl] B + H2O + N2\uparrow}\]
If acetaldehyde is treated with Fehling's solution, the change that occurs in the system is ____________.
Acetone reacts with iodine (I2) to form iodoform, in the presence of ____________.
The first oxidation product of secondary alcohols is ____________.
Which of the following carbonyl compounds does NOT undergo aldol condensation?
Which of the following is a Wolf - Kishner reduction?
The formation of cyanohydrin from acetone is an example of ____________.
\[\ce{CH2 = CH2 ->[i) O3][ii) Zn/H2O] X ->[NH3] Y}\] ‘Y’ is:
\[\ce{Ethanoic acid ->[P/Br2] 2-bromoethanoic acid}\]. This reaction is called ____________.
\[\ce{CH3Br ->[KNC] (A) ->[H3O^+] (B) ->[PCl5] (C)}\] product (C) is:
Which one of the following reaction is an example of disproportionation reaction.
Carboxylic acids have higher boiling points than aldehydes, ketones and even alcohols of comparable molecular mass. It is due to their ____________.
Identify A, B, C and D.
\[\ce{ethanoic acid ->[SOCl2] A ->[Pd/BaSO4] B ->[NaOH] C ->[][\Delta] D}\]
What is the action of HCN on ethanal?
How is the following conversion effected phenyl methanal into benzoin?
How will you prepare cinnamic acid from benzaldehyde?
The reagent used in Wolf - Kishner reduction is ______.
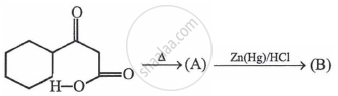
In the above reaction, product (B) is:
How are the following compound obtained from alkyne?
Acetone
Write reactions when phenol reacts with Concentrated HNO3
Write the structure of the major product of the aldol condensation of benzaldehyde with acetone.
What is the action of the following regent on propanal?
Sodium Bisulphite
Write the structure of the major product of the aldol condensation of benzaldehyde with acetone.
Write the structure of the major product of the aldol condensation of benzaldehyde with acetone.
Write the structure of the major product of the aldol condensation of benzaldehyde with acetone.
