Advertisements
Advertisements
Question
How will you prepare cinnamic acid from benzaldehyde?
Solution
Conversion of benzaldehyde into cinnamic acid:
\[\begin{array}{cc}
\phantom{....}\ce{COOH}\phantom{...............}\ce{COOH}\phantom{......}\\
\phantom{....}/\phantom{.....................}/\phantom{.............}\\
\ce{\underset{(Benzaldehyde)}{C6H5CH = O} + H2C ->[Pyridine][-H2O] C6H5CH = C ->[\Delta][-CO2] \underset{(Cinnamic acid)}{C6H5CH = CH} - COOH}\\
\phantom{....}\backslash\phantom{.....................}\backslash\phantom{.............}\\
\phantom{}\ce{\underset{(Malonic acid)}{COOH}}\phantom{.............}\ce{COOH}\phantom{....}
\end{array}\]
APPEARS IN
RELATED QUESTIONS
Write another name of disproportionation reaction?
The number of α-H atoms in butanal is ____________.
Which among the following compounds does NOT undergo aldol condensation?
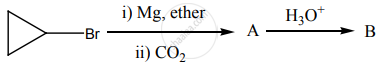
‘B’ is:
Identify A, B, C and D.
\[\ce{ethanoic acid ->[SOCl2] A ->[Pd/BaSO4] B ->[NaOH] C ->[][\Delta] D}\]
Complete the following reaction.
\[\begin{array}{cc}
\ce{CH3 - CH2 - CH2 - C - CH3 ->[HO - CH2 - CH2 - CH2 - OH][dry HCl] ?}\\
||\phantom{.........}\\
\ce{O}\phantom{.........}
\end{array}\]
Identify the reaction in which carbonyl group of aldehydes and ketones is reduced to methylene group on treatment with zinc-amalgam and cone. HCI.
Aldehydes are readily oxidised to yield carboxylic acids but ketones are inert to oxidation. Which is the most likely explanation regarding this difference in reactivity?
Write the structure of the major product of the aldol condensation of benzaldehyde with acetone.
Write the structure of the products obtained from the following ketones by action of hydrazine in presence of a slightly acidic medium.