Advertisements
Advertisements
Question
Identify A, B, C and D.
\[\ce{ethanoic acid ->[SOCl2] A ->[Pd/BaSO4] B ->[NaOH] C ->[][\Delta] D}\]
Solution
\[\begin{array}{cc}
\ce{\underset{(Ethanoic acid)}{CH3COOH} ->[SOCl2] \underset{\underset{(A)}{(Acetyl chloride)}}{CH3COCl} ->[Pd/BaSO4][H2] \underset{\underset{(B)}{(Acetaldehyde)}}{CH3CHO} + HCl ->[NaOH] \ce{CH3 - CH - CH2 - CHO} ->[-H2O][\Delta] \underset{\underset{(D)}{(Crotonaldehyde)}}{CH3 - CH = CH - CHO}}\\
\phantom{........................}|\\
\phantom{...........................}\ce{\underset{\underset{(C)}{(Aldol 3-hydroxy butanal)}}{OH}}
\end{array}\]
| A | CH3Cl | Acetyl chloride |
| B | CH3CHO | Acetaldehyde |
| C | \[\begin{array}{cc} \ce{CH3 - CH - CH2 - CHO}\\ |\phantom{..........}\\ \ce{OH}\phantom{.......} \end{array}\] |
3-hydroxy butanal |
| D | CH3 – CH = CH – CHO | Crotonaldehyde |
APPEARS IN
RELATED QUESTIONS
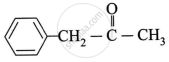
The product formed after reaction between benzaldehyde and methyl magnesium iodide followed by acid hydrolysis is ____________.
Which of the following is Schiff's reagent?
\[\ce{Benzoic acid ->[i) NH3][ii) \Delta] A ->[NaOBr] B ->[NaNO2/HCl] C}\] ‘C’ is:
Assertion: p-N, N-dimethyl amino benzaldehyde undergoes benzoin condensation
Reason: The aldehydic (−CHO) group is meta directing.
How will you convert benzaldehyde into the following compound?
α-hydroxy phenyl acetic acid
How will you prepare malachite green from benzaldehyde?
How acetone is converted into propane.
The reagent used in Wolf - Kishner reduction is ______.
Write a note on Clemmensen reduction.
Write the structure of the products obtained from the following ketones by action of hydrazine in presence of a slightly acidic medium.