Advertisements
Advertisements
Question
Write IUPAC name of the following
\[\begin{array}{cc}\ce{CH3-CH-CH-CH2-OH}\\|\phantom{.....}|\phantom{.......}\\\ce{OH}\phantom{..}\ce{CH3}\phantom{.....}\end{array}\]
Solution
2-Methylbutane-1,3-diol
APPEARS IN
RELATED QUESTIONS
What is metamerism?
Explain metamerism with suitable examples of ethers
Write the IUPAC name of the given compound:
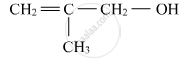
Name the following compound according to IUPAC system.
\[\begin{array}{cc}
\phantom{............}\ce{CH2OH}\\
\phantom{......}|\\
\ce{CH3 - CH2 - CH - CH - CH - CH3}\\
\phantom{......}|\phantom{............}|\phantom{.}\\
\phantom{........}\ce{CH2Cl}\phantom{......}\ce{CH3}\phantom{}
\end{array}\]
Name the following compound according to IUPAC system.
\[\begin{array}{cc}
\phantom{........................}\ce{CH2OH}\\
\phantom{..................}|\\
\ce{CH3 - CH - CH2 - CH - CH - CH3}\\
\phantom{}|\phantom{.............}|\phantom{........}\\
\phantom{..}\ce{CH3}\phantom{..........}\ce{OH}\phantom{........}
\end{array}\]
Name the following compound according to IUPAC system.
\[\begin{array}{cc}
\ce{H2C = CH - CH - CH2 - CH2 - CH3}\\
|\phantom{..........}\\
\ce{OH}\phantom{........}
\end{array}\]
Name the following compound according to IUPAC system.
\[\begin{array}{cc}
\ce{CH3 - C = C - CH2OH}\\
\phantom{}|\phantom{....}|\phantom{....}\\
\phantom{}\ce{CH3}\phantom{.}\ce{Br}\phantom{...}
\end{array}\]
Write IUPAC name of the following compound:
\[\begin{array}{cc}
\phantom{................}\ce{CH3}\\
\phantom{.............}|\\
\ce{CH3 - CH - CH - C - CH3}\\
|\phantom{......}|\phantom{......}|\\
\phantom{...}\ce{CH3\phantom{.}}\phantom{..}\ce{OH}\phantom{...}\ce{CH3}
\end{array}\]
Write IUPAC name of the following compound:
\[\begin{array}{cc}
\ce{H3C - CH - CH2 - CH - CH - CH2 - CH3}\\
\phantom{}|\phantom{.............}|\phantom{......}|\phantom{.........}\\
\phantom{}\ce{OH}\phantom{..........}\ce{OH}\phantom{...}\ce{C2H5}\phantom{......}
\end{array}\]
Write IUPAC name of the following compound:
\[\begin{array}{cc}
\ce{CH3 - CH - CH - CH3}\\
|\phantom{......}|\phantom{..}\\
\ce{OH}\phantom{...}\ce{OH}\phantom{}
\end{array}\]
Write IUPAC name of the following compound:
\[\begin{array}{cc}
\ce{HO - CH2 - CH - CH2 - OH}\\
|\phantom{..}\\
\ce{OH}
\end{array}\]
Write IUPAC name of the following compound:
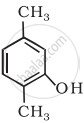
Write IUPAC name of the following compound:
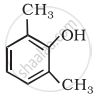
Write IUPAC name of the following compound:
C6H5 – O – C2H5
Write IUPAC name of the following compound:
C6H5 – O – C7H15(n−)
Write IUPAC name of the following compound:
\[\begin{array}{cc}
\ce{CH3 - CH2 - O - CH - CH2 - CH3}\\
\phantom{...}|\\
\phantom{.....}\ce{CH3}
\end{array}\]
Write structures of the compounds whose IUPAC names are as follows:
3-Chloromethylpentan-1-ol.
- Draw the structures of all isomeric alcohols of molecular formula C5H12O and give their IUPAC names.
- Classify the isomers of alcohols in the above question as primary, secondary and tertiary alcohols.
Give IUPAC name of the following ether:
\[\begin{array}{cc}
\ce{C2H5OCH2 - CH - CH3}\\
\phantom{.....}|\\
\phantom{.......}\ce{CH3}
\end{array}\]
Give IUPAC name of the following ether:
CH3OCH2CH2Cl
Give IUPAC name of the following ether:
O2N – C6H4 – OCH3(p)
Give IUPAC name of the following ether:
CH3CH2CH2OCH3
Ethylidene dichloride when boiled with aqueous solution of NaOH yields _______.
(A) formaldehyde
(B) acetaldehyde
(C) acetone
(D) ethyl methyl ketone
Which of the following compounds is NOT prepared by the action of alcoholic NI3 on alkyl halide?
(a) CH3NH2
(b) CH3- CH2- NH2
(c) CH3 - CH2 - CH2 - NH2
(d) (CH3)3 C- NH2
How is phenol converted into the following?
benzoquinone
How is phenol converted into the following?
picric acid
Write IUPAC name of the following compound (CH3)2 N − CH2CH3
Write the structures of the products when Butan-2-ol reacts with CrO3
Write the structures of the products when Butan-2-ol reacts with SOCl2
In the dehydration of alcohols to alkenes by heating with concentrated sulphuric acid, the initiation step is:
(1) formation of carbonation
(2) formation of an ester
(3) protonation of the alcohol molecule
(4) elimination of water
Write structural formulae for 1-Ethylcyclohexanol.
Write structural formulae for Pentane-1,4-diol
Write structural formulae for Cyclohex-2-en-1-ol.
Write IUPAC name of the following
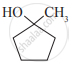
Write IUPAC names of the following
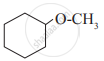
In a carbinol system of nomenclature tert.butyl alcohol is named as _______________
Glycerol is ____________.
Isopropyl alcohol on oxidation forms:
Give IUPAC names of the following compound:
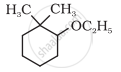
Give IUPAC names of the following compound:
\[\begin{array}{cc}
\phantom{..}\ce{H}\phantom{...}\ce{CH3}\phantom{.}\ce{H}\phantom{..}\\
\phantom{}|\phantom{....}|\phantom{....}|\phantom{}\\
\ce{H - C - C - C - H}\\
\phantom{}|\phantom{....}|\phantom{....}|\phantom{}\\
\phantom{.}\ce{H}\phantom{...}\ce{OH}\phantom{.}\ce{H}\phantom{.}\\
\end{array}\]
C6H5OCH2CH3 is called:
One of the following is not a dihydroxy derivative of benzene.
An example of a compound with functional group – O – is ____________.
Ethyl alcohol is industrially prepared from ethylene by:
IUPAC name of m-cresol is ____________.
Ethylene reacts with Baeyer’s reagent to give ______.
When ethyl alcohol reacts with acetic acid, the products formed are:
1-Propanol and 2-propanol can be best distinguished by:
Which of the following is most acidic?
The IUPAC name of the ether CH2 = CH–CH2OCH3 is:
The heating of phenyl methyl ether with HI produces:
Among the following sets of reactants which one produces anisole?
IUPAC name of the compound is:
\[\begin{array}{cc}
\ce{CH3-CH-OCH3}\\
|\phantom{....}\\
\ce{CH3}\phantom{..}
\end{array}\]
The correct acidic strength order of the following is:
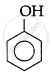
(I)

(II)
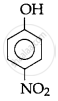
(III)
IUPAC name of the compound \[\begin{array}{cc}
\ce{CH3 - CH - OCH3}\\
\phantom{}|\phantom{....}\\
\phantom{}\ce{CH3}\phantom{..}
\end{array}\] is ______.
Write the IUPAC name of the compound given below.
\[\begin{array}{cc}
\phantom{}\ce{CH3 - CH2 - C = C - OH}\\
\phantom{........}|\phantom{....}|\phantom{}\\
\phantom{..............}\ce{CH3 CH2OH}
\end{array}\]
Write steps to carry out the conversion of phenol to aspirin.
Explain why p-nitrophenol is more acidic than phenol.
Match the structures of the compounds given in Column I with the name of the compounds given in Column II.
| Column I | Column II | |
| (i) | 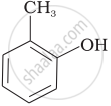 |
(a) Hydroquinone |
| (ii) | 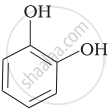 |
(b) Phenetole |
| (iii) | 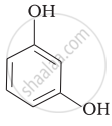 |
(c) Catechol |
| (iv) | 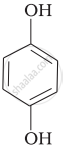 |
(d) o-Cresol |
| (v) | 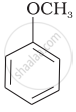 |
(e) guinone |
| (vi) | 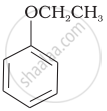 |
(f) Resorcinol |
| (g) Anisole |
Match the starting materials given in Column I with the products formed by these (Column II) in the reaction with HI.
| Column I | Column II | ||
| (i) | CH3—O—CH3 | (a) | 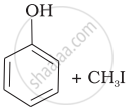 |
| (ii) | \[\begin{array}{cc} \ce{CH3}\phantom{..................}\\ \backslash\phantom{.............}\\ \ce{CH-O-CH3}\\ /\phantom{..............}\\ \ce{CH3}\phantom{..................} \end{array}\] |
(b) | \[\begin{array}{cc} \ce{CH3}\phantom{....}\\ |\phantom{.......}\\ \ce{CH3-C-I + CH3OH}\\ |\phantom{.......}\\ \ce{CH3}\phantom{....} \end{array}\] |
| (iii) | \[\begin{array}{cc} \ce{CH3}\phantom{.}\\ |\phantom{....}\\ \ce{H3C-C-O-CH3}\\ |\phantom{....}\\ \ce{CH3}\phantom{..} \end{array}\] |
(c) | 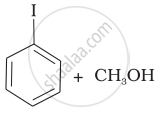 |
| (iv) | 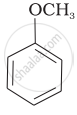 |
(d) | CH3—OH + CH3—I |
| (e) | \[\begin{array}{cc} \ce{CH3}\phantom{.....................}\\ \backslash\phantom{.................}\\ \ce{CH-OH + CH3I}\\ /\phantom{.................}\\ \ce{CH3}\phantom{.....................} \end{array}\] |
||
| (f) | \[\begin{array}{cc} \ce{CH3}\phantom{.....................}\\ \backslash\phantom{.................}\\ \ce{CH-I + CH3OH}\\ /\phantom{.................}\\ \ce{CH3}\phantom{.....................} \end{array}\] |
||
| (g) | \[\begin{array}{cc} \ce{CH3}\phantom{....}\\ |\phantom{.......}\\ \ce{CH3-C-OH + CH3I}\\ |\phantom{.......}\\ \ce{CH3}\phantom{....} \end{array}\] |
Assertion: p-nitrophenol is more acidic than phenol.
Reason: Nitro group helps in the stabilisation of the phenoxide ion by dispersal of negative charge due to resonance.
Assertion: IUPAC name of the compound
\[\begin{array}{cc}
\ce{CH3 - CH - O - CH2 - CH2 - CH3}\\
|\phantom{....................}\\
\ce{CH3}\phantom{.................}
\end{array}\] is 2-Ethoxy-2-methylethane.
Reason: In IUPAC nomenclature, ether is regarded as hydrocarbon derivative in which a hydrogen atom is replaced by —OR or —OAr group [where R = alkyl group and Ar = aryl group]
Assertion: Phenol forms 2, 4, 6 – tribromophenol on treatment with \[\ce{Br2}\] in carbon disulphide at 273 K.
Reason: Bromine polarises in carbon disulphide.
Assertion: Phenols give o- and p-nitrophenol on nitration with conc. \[\ce{HNO3}\] and \[\ce{H2SO4}\] mixture.
Reason: –OH group in phenol is o–, p– directing.
How can phenol be converted to aspirin?
Write chemical reactions for the following conversion:
Acetic acid into ethyl alcohol
Identify A and B in the following:

Write IUPAC name of the following compound:
\[\begin{array}{cc}
\phantom{................}\ce{CH3}\\
\phantom{.............}|\\
\ce{CH3 - CH - CH - C - CH3}\\
|\phantom{......}|\phantom{......}|\\
\phantom{...}\ce{CH3}\phantom{..}\ce{OH}\phantom{...}\ce{CH3}
\end{array}\]
Draw structure of the following compound.
2-Methoxypropane
Draw structure of the following compound.
Prop-2-en-1-ol
Give the structures of Thiosulphuric acid and Peroxy monosulphuric acid.
Write structural formulae for:
p-Nitrophenol
Write structural formulae for:
Salicylic acid
The IUPAC name of  is ______.
is ______.
Write IUPAC name of the following compound:
\[\begin{array}{cc}
\phantom{...............}\ce{CH3}\\
\phantom{............}|\\
\ce{CH3 - CH - CH - C - CH3}\\
|\phantom{......}|\phantom{......}|\\
\phantom{...}\ce{CH3}\phantom{...}\ce{OH}\phantom{...}\ce{CH3}
\end{array}\]
Write the IUPAC name.
\[\begin{array}{cc}
\phantom{................}\ce{CH3}\\
\phantom{.............}|\\
\ce{CH3 - CH - CH - C -CH3}\\
\phantom{.}|\phantom{......}|\phantom{......}|\\
\phantom{....}\ce{CH3\phantom{...}\ce{OH}\phantom{...}\ce{CH3}}\
\end{array}\]
Write IUPAC names of the following compounds:
\[\begin{array}{cc}
\phantom{...............}\ce{CH3}\\
\phantom{.............}|\\
\ce{CH3 - CH - CH - C - CH3}\\
|\phantom{......}|\phantom{......}|\\
\phantom{...}\ce{CH3\phantom{...}OH\phantom{...}CH3}\\
\end{array}\]
Write the IUPAC name of the following compound:
\[\begin{array}{cc}
\phantom{...............}\ce{CH3}\\
\phantom{.............}|\\
\ce{CH3 - CH - CH - C - CH3}\\
|\phantom{......}|\phantom{......}|\\
\phantom{...}\ce{CH3}\phantom{..}\ce{OH}\phantom{...}\ce{CH3}
\end{array}\]
