Advertisements
Advertisements
Question
Write IUPAC name of the following compound:
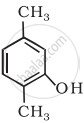
Solution
2, 5-Dimethylphenol
APPEARS IN
RELATED QUESTIONS
Name the following compound according to IUPAC system.
\[\begin{array}{cc}
\phantom{............}\ce{CH2OH}\\
\phantom{......}|\\
\ce{CH3 - CH2 - CH - CH - CH - CH3}\\
\phantom{......}|\phantom{............}|\phantom{.}\\
\phantom{........}\ce{CH2Cl}\phantom{......}\ce{CH3}\phantom{}
\end{array}\]
Name the following compound according to IUPAC system.
\[\begin{array}{cc}
\ce{CH3 - C = C - CH2OH}\\
\phantom{}|\phantom{....}|\phantom{....}\\
\phantom{}\ce{CH3}\phantom{.}\ce{Br}\phantom{...}
\end{array}\]
Write IUPAC name of the following compound:
\[\begin{array}{cc}
\ce{CH3 - CH - CH - CH3}\\
|\phantom{......}|\phantom{..}\\
\ce{OH}\phantom{...}\ce{OH}\phantom{}
\end{array}\]
- Draw the structures of all isomeric alcohols of molecular formula C5H12O and give their IUPAC names.
- Classify the isomers of alcohols in the above question as primary, secondary and tertiary alcohols.
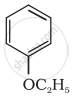
Give IUPAC name of the following ether:
CH3OCH2CH2Cl
Give IUPAC name of the following ether:
Ethylidene dichloride when boiled with aqueous solution of NaOH yields _______.
(A) formaldehyde
(B) acetaldehyde
(C) acetone
(D) ethyl methyl ketone
Write the structure and IUPAC name of 'methyl-n-propyl ether'.
3-Methylbutane-2-ol on heating with HI gives ______
What.will be the product fonned when chlorobenzene is heated with sodium metal in the presence of dry ether?
Write IUPAC names of the following
C6H5OCH2CH3 is called:
An example of a compound with functional group – O – is ____________.
n-Propyl alcohol and isopropyl alcohol can be chemically distinguished by which reagent?
Write the IUPAC name of the compound given below.
\[\begin{array}{cc}
\phantom{}\ce{CH3 - CH2 - C = C - OH}\\
\phantom{........}|\phantom{....}|\phantom{}\\
\phantom{..............}\ce{CH3 CH2OH}
\end{array}\]
Arrange the following compounds in decreasing order of acidity.
\[\ce{H2O, ROH, HC ≡ CH}\]
Write steps to carry out the conversion of phenol to aspirin.
Explain why p-nitrophenol is more acidic than phenol.
Draw structure of the following compound.
Prop-2-en-1-ol
The IUPAC name of 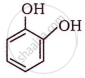 is ______.
is ______.
