Advertisements
Advertisements
Question
Write IUPAC name of the following compound:
\[\begin{array}{cc}
\ce{HO - CH2 - CH - CH2 - OH}\\
|\phantom{..}\\
\ce{OH}
\end{array}\]
Solution
Propane-1, 2, 3-triol
APPEARS IN
RELATED QUESTIONS
What is metamerism?
Write IUPAC name of the following compound:
C6H5 – O – C2H5
Write IUPAC name of the following compound:
C6H5 – O – C7H15(n−)
Write structures of the compounds whose IUPAC names are as follows:
3-Chloromethylpentan-1-ol.
Give IUPAC name of the following ether:
\[\begin{array}{cc}
\ce{C2H5OCH2 - CH - CH3}\\
\phantom{.....}|\\
\phantom{.......}\ce{CH3}
\end{array}\]
Give reasons Fluoride ion has higher hydration enthalpy than chloride ion.
Write the IUPAC name of the following :
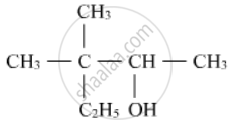
Write IUPAC name of the following
\[\begin{array}{cc}\ce{CH3-CH-CH-CH2-OH}\\|\phantom{.....}|\phantom{.......}\\\ce{OH}\phantom{..}\ce{CH3}\phantom{.....}\end{array}\]
3-methylphenol is called ____________.
The compound HOCH2 – CH2OH is __________.
The IUPAC name of the ether CH2 = CH–CH2OCH3 is:
Which of the following reagents can be used to oxidise primary alcohols to aldehydes?
(i) \[\ce{CrO3}\] in anhydrous medium.
(ii) \[\ce{KMnO4}\] in acidic medium.
(iii) Pyridinium chlorochromate.
(iv) Heat in the presence of Cu at 573 K.
What happens when benzene diazonium chloride is heated with water?
Arrange the following compounds in decreasing order of acidity.
\[\ce{H2O, ROH, HC ≡ CH}\]
Write complete reaction for the bromination of phenol in aqueous and non-aqueous medium.
How can phenol be converted to aspirin?
Convert the following:
Ethyl alcohol into ethyl acetate
Give the structures of Thiosulphuric acid and Peroxy monosulphuric acid.
Write the IUPAC name of the following compound:
\[\begin{array}{cc}
\phantom{..............}\ce{CH3}\\
\phantom{............}|\\
\ce{CH3 - CH - CH - C - CH3}\\
|\phantom{.....}|\phantom{......}|\\
\phantom{...}\ce{CH3}\phantom{.}\ce{OH}\phantom{...}\ce{CH3}
\end{array}\]
Write IUPAC name of the following compound:
\[\begin{array}{cc}
\phantom{................}\ce{CH3}\\
\phantom{.............}|\\
\ce{CH3 - CH - CH - C - CH3}\\
|\phantom{......}|\phantom{......}|\\
\phantom{...}\ce{CH3}\phantom{...}\ce{OH}\phantom{...}\ce{CH3}
\end{array}\]
