Advertisements
Advertisements
प्रश्न
Name the following compound according to IUPAC system.
\[\begin{array}{cc}
\ce{CH3 - C = C - CH2OH}\\
\phantom{}|\phantom{....}|\phantom{....}\\
\phantom{}\ce{CH3}\phantom{.}\ce{Br}\phantom{...}
\end{array}\]
उत्तर
2-Bromo-3-methylbut-2-en-1-ol
APPEARS IN
संबंधित प्रश्न
Name the following compound according to IUPAC system.
\[\begin{array}{cc}
\phantom{........................}\ce{CH2OH}\\
\phantom{..................}|\\
\ce{CH3 - CH - CH2 - CH - CH - CH3}\\
\phantom{}|\phantom{.............}|\phantom{........}\\
\phantom{..}\ce{CH3}\phantom{..........}\ce{OH}\phantom{........}
\end{array}\]
Write IUPAC name of the following compound:
\[\begin{array}{cc}
\phantom{................}\ce{CH3}\\
\phantom{.............}|\\
\ce{CH3 - CH - CH - C - CH3}\\
|\phantom{......}|\phantom{......}|\\
\phantom{...}\ce{CH3\phantom{.}}\phantom{..}\ce{OH}\phantom{...}\ce{CH3}
\end{array}\]
Write IUPAC name of the following compound:
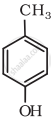
Write IUPAC name of the following compound:
C6H5 – O – C2H5
Give IUPAC name of the following ether:
\[\begin{array}{cc}
\ce{C2H5OCH2 - CH - CH3}\\
\phantom{.....}|\\
\phantom{.......}\ce{CH3}
\end{array}\]
Give reasons Fluoride ion has higher hydration enthalpy than chloride ion.
What.will be the product fonned when chlorobenzene is heated with sodium metal in the presence of dry ether?
Resorcinol on distillation with zinc dust gives _________.
C6H5OCH2CH3 is called:
The compound HOCH2 – CH2OH is __________.
Butane-2-ol is ____________.
IUPAC name of m-cresol is ____________.
Ethylene reacts with Baeyer’s reagent to give ______.
1-Propanol and 2-propanol can be best distinguished by:
Which of the following gives a positive iodoform test?
Among the following sets of reactants which one produces anisole?
Give IUPAC name of the compound given below.
\[\begin{array}{cc}
\phantom{}\ce{CH3 - CH - CH2 - CH2 - CH - CH3}\phantom{.}\\
\phantom{.........}|\phantom{...................}|\phantom{...........}\\
\phantom{..}\ce{Cl}\phantom{.................}\ce{OH}\phantom{..}
\end{array}\]
Assertion: Phenol forms 2, 4, 6 – tribromophenol on treatment with \[\ce{Br2}\] in carbon disulphide at 273 K.
Reason: Bromine polarises in carbon disulphide.
Write IUPAC name of the following compound:
\[\begin{array}{cc}
\phantom{................}\ce{CH3}\\
\phantom{.............}|\\
\ce{CH3 - CH - CH - C - CH3}\\
|\phantom{......}|\phantom{......}|\\
\phantom{...}\ce{CH3}\phantom{..}\ce{OH}\phantom{...}\ce{CH3}
\end{array}\]
Give the structures of Thiosulphuric acid and Peroxy monosulphuric acid.
