Advertisements
Advertisements
Question
Match the items of column I with items of column II.
| Column I | Column II | |
| (i) | Antifreeze used in car engine | (a) Neutral ferric chloride |
| (ii) | Solvent used in perfumes | (b) Glycerol |
| (iii) | Starting material for picric acid | (c) Methanol |
| (iv) | Wood spirit | (d) Phenol |
| (v) | Reagent used for detection of phenolic group | (e) Ethleneglycol |
| (vi) | By product of soap industry used in cosmetics | (f) Ethanol |
Solution
| Column I | Column II | |
| (i) | Antifreeze used in car engine | (e) Ethleneglycol |
| (ii) | Solvent used in perfumes | (f) Ethanol |
| (iii) | Starting material for picric acid | (d) Phenol |
| (iv) | Wood spirit | (c) Methanol |
| (v) | Reagent used for detection of phenolic group | (a) Neutral ferric chloride |
| (vi) | By product of soap industry used in cosmetics | (b) Glycerol |
Explanation:
(i) IUPAC name of ethylene glycol is ethane-1, 2-diol. It is primarily used as raw material in the manufacturing of polyester fibers and fabric industry. A small percentage of it is used in antifreeze formulations.
(ii) Ethanol is a good solvent for fatty and waxy substances. Fats and waxes provide odour to the perfumes. Apart from being a good solvent, it is less irritating to the skin. So, it is used in perfumes.
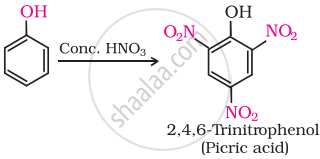
(iii) Phenol is converted into picric acid (2, 4, 6-trinitro-phenol) by the reaction of phenol with cone. \[\ce{HNO3}\].
(iv) Methanol, \[\ce{CH3OH}\] is also known as ‘wood spirit’ as it was produced by the destructive distillation of wood.
(v) Neutral ferric chloride give purple/red colour when treated with phenols. It is the reagent used for detection of phenolic group.
(vi) Soaps are prepared by the reactions of fatty acid with \[\ce{NaOH}\].
\[\begin{array}{cc}
\ce{O}\phantom{..........................................}\\
||\phantom{..........................................}\\
\ce{CH2 - O - C - OC17H35}\phantom{...........................}\phantom{.......}\ce{CH2OH}\phantom{.}\\
\phantom{.}
\phantom{.........}|\phantom{..........}\ce{O}\phantom{.............................................}|\phantom{.................}\\
||\phantom{...........................................}\\
\ce{CH - O - C - OC17H35 + 3NaOH -> \underset{(soap)}{\underset{Sodium stearate}{3C17H35COONa}} + CHOH}\phantom{..}\\
\phantom{..}|\phantom{.........................................................}|\phantom{.........}\\
\phantom{}\ce{CH2 - O - C -OC17H35}\phantom{..................................}\ce{\underset{Glycerol}{CH2OH}}\phantom{}\\
||\phantom{........................................}\\
\ce{O}\phantom{........................................}\\
\end{array}\]
The glycerol (propan-1, 2, 3-triol) is the by product of saop industry and used in cosmetics.
APPEARS IN
RELATED QUESTIONS
Write the mechanism of the following reaction :
\[\ce{C2H5OH->[H2SO4][443K]CH2=CH2 + H2O}\]
Which of the following is correct?
Arrange the following compounds in increasing order of acidity and give a suitable explanation.
Phenol, o-nitrophenol, o-cresol
The carbon-oxygen bond in phenol is slightly stronger than that in methanol. Why?
Match the items of column I with items of column II.
| Column I | Column II | |
| (i) | Methanol | (a) Conversion of phenol to o-hydroxysalicylic acid |
| (ii) | Kolbe’s reaction | (b) Ethyl alcohol |
| (iii) | Williamson’s synthesis | (c) Conversion of phenol to salicylaldehyde |
| (iv) | Conversion of 2° alcohol to ketone | (d) Wood spirit |
| (v) | Reimer-Tiemann reaction | (e) Heated copper at 573 K |
| (vi) | Fermentation | (f) Reaction of alkyl halide with sodium alkoxide |
Convert the following:
Propanenitrile to ethanol.
Tonics in general contain
Which of the following is known as wood spirit?
Write chemical reactions of following reagents on methoxyethane:
dilute H2SO4
How methanol is obtained from methanal.
