Advertisements
Advertisements
Question
Arrange the following compounds in increasing order of acidity and give a suitable explanation.
Phenol, o-nitrophenol, o-cresol
Solution
Increasing order of acidity.
o-cresol < Phenol < o-nitrophenol
In cresol, 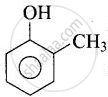 , the electron-donating group \[\ce{(-CH3)}\] gives electrons and intensify the charge on phenoxide ion and therefore makes it unstable. Therefore, o-cresol is less acidic than phenol. In o-nitrophenol, the electron-withdrawing \[\ce{(-NO2)}\] group withdraws electrons and disperses the - ve charge and stabilizes the phenoxide ion. Therefore, o-nitrophenol is more acidic than phenol.
, the electron-donating group \[\ce{(-CH3)}\] gives electrons and intensify the charge on phenoxide ion and therefore makes it unstable. Therefore, o-cresol is less acidic than phenol. In o-nitrophenol, the electron-withdrawing \[\ce{(-NO2)}\] group withdraws electrons and disperses the - ve charge and stabilizes the phenoxide ion. Therefore, o-nitrophenol is more acidic than phenol.
APPEARS IN
RELATED QUESTIONS
Methanol and ethanol are miscible in water due to ____________.
If ethanol dissolves in water, then which of the following would be observed:
Which of the following is correct?
Phenol can be distinguished from ethanol by the reactions with:
(i) \[\ce{Br2/water}\]
(ii) \[\ce{Na}\]
(iii) Neutral \[\ce{FeCl3}\]
(iv) All the above
The carbon-oxygen bond in phenol is slightly stronger than that in methanol. Why?
Arrange water, ethanol and phenol in increasing order of acidity and give reason for your answer.
Match the items of column I with items of column II.
| Column I | Column II | |
| (i) | Methanol | (a) Conversion of phenol to o-hydroxysalicylic acid |
| (ii) | Kolbe’s reaction | (b) Ethyl alcohol |
| (iii) | Williamson’s synthesis | (c) Conversion of phenol to salicylaldehyde |
| (iv) | Conversion of 2° alcohol to ketone | (d) Wood spirit |
| (v) | Reimer-Tiemann reaction | (e) Heated copper at 573 K |
| (vi) | Fermentation | (f) Reaction of alkyl halide with sodium alkoxide |
Convert the following:
Propanenitrile to ethanol.
Which of the following is known as wood spirit?
How methanol is obtained from methanal.
