Advertisements
Advertisements
प्रश्न
Arrange the following compounds in increasing order of acidity and give a suitable explanation.
Phenol, o-nitrophenol, o-cresol
उत्तर
Increasing order of acidity.
o-cresol < Phenol < o-nitrophenol
In cresol, 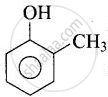 , the electron-donating group \[\ce{(-CH3)}\] gives electrons and intensify the charge on phenoxide ion and therefore makes it unstable. Therefore, o-cresol is less acidic than phenol. In o-nitrophenol, the electron-withdrawing \[\ce{(-NO2)}\] group withdraws electrons and disperses the - ve charge and stabilizes the phenoxide ion. Therefore, o-nitrophenol is more acidic than phenol.
, the electron-donating group \[\ce{(-CH3)}\] gives electrons and intensify the charge on phenoxide ion and therefore makes it unstable. Therefore, o-cresol is less acidic than phenol. In o-nitrophenol, the electron-withdrawing \[\ce{(-NO2)}\] group withdraws electrons and disperses the - ve charge and stabilizes the phenoxide ion. Therefore, o-nitrophenol is more acidic than phenol.
APPEARS IN
संबंधित प्रश्न
Write resonance structures of aniline. What is the action of benzene diazonium chloride on ethanol?
How do you convert the following :
Ethanol to Propan-2-ol
Write the mechanism of the following reaction :
\[\ce{C2H5OH->[H2SO4][443K]CH2=CH2 + H2O}\]
Wood spirit is known as acetone:
Write the IUPAC name of the following compound.
\[\begin{array}{cc}
\phantom{.}\ce{CH3 - CH - CH - CH - CH - CH3}\phantom{}\\
\phantom{......}|\phantom{......}|\phantom{......}|\phantom{.....}|\phantom{........}\\
\phantom{...}\ce{CH3}\phantom{...}\ce{OH}\phantom{...}\ce{C2H5}\phantom{.}\ce{OH}\phantom{...}\end{array}\]
What is denatured alcohol?
Suggest a reagent for conversion of ethanol to ethanoic acid.
Tonics in general contain
Write chemical reactions of following reagents on methoxyethane:
dilute H2SO4
How methanol is obtained from methanal.
