Advertisements
Advertisements
प्रश्न
Arrange the following compounds in increasing order of acidity and give a suitable explanation.
Phenol, o-nitrophenol, o-cresol
उत्तर
Increasing order of acidity.
o-cresol < Phenol < o-nitrophenol
In cresol, 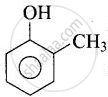 , the electron-donating group \[\ce{(-CH3)}\] gives electrons and intensify the charge on phenoxide ion and therefore makes it unstable. Therefore, o-cresol is less acidic than phenol. In o-nitrophenol, the electron-withdrawing \[\ce{(-NO2)}\] group withdraws electrons and disperses the - ve charge and stabilizes the phenoxide ion. Therefore, o-nitrophenol is more acidic than phenol.
, the electron-donating group \[\ce{(-CH3)}\] gives electrons and intensify the charge on phenoxide ion and therefore makes it unstable. Therefore, o-cresol is less acidic than phenol. In o-nitrophenol, the electron-withdrawing \[\ce{(-NO2)}\] group withdraws electrons and disperses the - ve charge and stabilizes the phenoxide ion. Therefore, o-nitrophenol is more acidic than phenol.
APPEARS IN
संबंधित प्रश्न
Write the mechanism of the following reaction :
\[\ce{C2H5OH->[H2SO4][443K]CH2=CH2 + H2O}\]
If ethanol dissolves in water, then which of the following would be observed:
Wood spirit is known as acetone:
Phenol is less acidic than ______.
Phenol can be distinguished from ethanol by the reactions with:
(i) \[\ce{Br2/water}\]
(ii) \[\ce{Na}\]
(iii) Neutral \[\ce{FeCl3}\]
(iv) All the above
Write the IUPAC name of the following compound.
\[\begin{array}{cc}
\phantom{.}\ce{CH3 - CH - CH - CH - CH - CH3}\phantom{}\\
\phantom{......}|\phantom{......}|\phantom{......}|\phantom{.....}|\phantom{........}\\
\phantom{...}\ce{CH3}\phantom{...}\ce{OH}\phantom{...}\ce{C2H5}\phantom{.}\ce{OH}\phantom{...}\end{array}\]
Suggest a reagent for conversion of ethanol to ethanal.
The carbon-oxygen bond in phenol is slightly stronger than that in methanol. Why?
Convert the following Ethanal to ethanol.
How methanol is obtained from methanal.
