Advertisements
Advertisements
प्रश्न
The carbon-oxygen bond in phenol is slightly stronger than that in methanol. Why?
उत्तर
This can be explained as under:
(i) In phenol, the conjugation of unshared electron pairs over oxygen with aromatic ring results in partial double bond character in C – O bond.
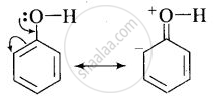
In methanol, no such conjugation (resonance) is possible.
(ii) In phenol, oxygen is attached to sp2 hybridised carbon while in methanol, oxygen attached to sp2 hybridised carbon. An sp2 hybridised carbon is more electronegative (because of greater 5-character) than sp3 hybridised carbon atom. Therefore, the bond between oxygen and sp2 hybridised carbon is more stable than the bond between oxygen and sp2, hybridised orbital.
APPEARS IN
संबंधित प्रश्न
Among the following the one which reacts most readily with ethanol is:
Which of the following is correct?
Phenol is less acidic than ______.
What is denatured alcohol?
Out of 2-chloroethanol and ethanol which is more acidic and why?
Arrange the following compounds in increasing order of acidity and give a suitable explanation.
Phenol, o-nitrophenol, o-cresol
Tonics in general contain
Write chemical reactions of following reagents on methoxyethane:
dilute H2SO4
Convert the following Ethanal to ethanol.
Give IUPAC names of the following compounds:
\[\begin{array}{cc}
\ce{CH3 - CH - CH - CH - CH2-OH }\\
|\phantom{......}|\phantom{......}|\phantom{.......}\\
\ce{Cl}\phantom{....}\ce{CH3}\phantom{...}\ce{CH3}\phantom{.....}
\end{array}\]
