Advertisements
Advertisements
Question
The carbon-oxygen bond in phenol is slightly stronger than that in methanol. Why?
Solution
This can be explained as under:
(i) In phenol, the conjugation of unshared electron pairs over oxygen with aromatic ring results in partial double bond character in C – O bond.
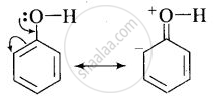
In methanol, no such conjugation (resonance) is possible.
(ii) In phenol, oxygen is attached to sp2 hybridised carbon while in methanol, oxygen attached to sp2 hybridised carbon. An sp2 hybridised carbon is more electronegative (because of greater 5-character) than sp3 hybridised carbon atom. Therefore, the bond between oxygen and sp2 hybridised carbon is more stable than the bond between oxygen and sp2, hybridised orbital.
APPEARS IN
RELATED QUESTIONS
Write resonance structures of aniline. What is the action of benzene diazonium chloride on ethanol?
If ethanol dissolves in water, then which of the following would be observed:
Wood spirit is known as acetone:
Arrange the following compounds in increasing order of acidity and give a suitable explanation.
Phenol, o-nitrophenol, o-cresol
Assertion: Ethanol is a weaker acid than phenol.
Reason: Sodium ethoxide may be prepared by the reaction of ethanol with aqueous \[\ce{NaOH}\].
Convert the following:
Propanenitrile to ethanol.
Tonics in general contain
Alcoholic fermentation is brought about by the action of
Which of the following is known as wood spirit?
If the starting material is 1-methyl-1,2-epoxy cyclopentane, of absolute configuration, decide which one compound correctly represents the product of its reaction with sodium methoxide in methanol.
