Advertisements
Advertisements
प्रश्न
Match the items of column I with items of column II.
| Column I | Column II | |
| (i) | Antifreeze used in car engine | (a) Neutral ferric chloride |
| (ii) | Solvent used in perfumes | (b) Glycerol |
| (iii) | Starting material for picric acid | (c) Methanol |
| (iv) | Wood spirit | (d) Phenol |
| (v) | Reagent used for detection of phenolic group | (e) Ethleneglycol |
| (vi) | By product of soap industry used in cosmetics | (f) Ethanol |
उत्तर
| Column I | Column II | |
| (i) | Antifreeze used in car engine | (e) Ethleneglycol |
| (ii) | Solvent used in perfumes | (f) Ethanol |
| (iii) | Starting material for picric acid | (d) Phenol |
| (iv) | Wood spirit | (c) Methanol |
| (v) | Reagent used for detection of phenolic group | (a) Neutral ferric chloride |
| (vi) | By product of soap industry used in cosmetics | (b) Glycerol |
Explanation:
(i) IUPAC name of ethylene glycol is ethane-1, 2-diol. It is primarily used as raw material in the manufacturing of polyester fibers and fabric industry. A small percentage of it is used in antifreeze formulations.
(ii) Ethanol is a good solvent for fatty and waxy substances. Fats and waxes provide odour to the perfumes. Apart from being a good solvent, it is less irritating to the skin. So, it is used in perfumes.
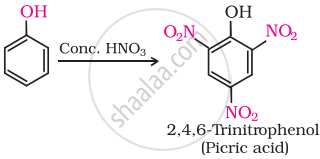
(iii) Phenol is converted into picric acid (2, 4, 6-trinitro-phenol) by the reaction of phenol with cone. \[\ce{HNO3}\].
(iv) Methanol, \[\ce{CH3OH}\] is also known as ‘wood spirit’ as it was produced by the destructive distillation of wood.
(v) Neutral ferric chloride give purple/red colour when treated with phenols. It is the reagent used for detection of phenolic group.
(vi) Soaps are prepared by the reactions of fatty acid with \[\ce{NaOH}\].
\[\begin{array}{cc}
\ce{O}\phantom{..........................................}\\
||\phantom{..........................................}\\
\ce{CH2 - O - C - OC17H35}\phantom{...........................}\phantom{.......}\ce{CH2OH}\phantom{.}\\
\phantom{.}
\phantom{.........}|\phantom{..........}\ce{O}\phantom{.............................................}|\phantom{.................}\\
||\phantom{...........................................}\\
\ce{CH - O - C - OC17H35 + 3NaOH -> \underset{(soap)}{\underset{Sodium stearate}{3C17H35COONa}} + CHOH}\phantom{..}\\
\phantom{..}|\phantom{.........................................................}|\phantom{.........}\\
\phantom{}\ce{CH2 - O - C -OC17H35}\phantom{..................................}\ce{\underset{Glycerol}{CH2OH}}\phantom{}\\
||\phantom{........................................}\\
\ce{O}\phantom{........................................}\\
\end{array}\]
The glycerol (propan-1, 2, 3-triol) is the by product of saop industry and used in cosmetics.
APPEARS IN
संबंधित प्रश्न
How do you convert the following :
Ethanol to Propan-2-ol
Methanol and ethanol are miscible in water due to ____________.
If ethanol dissolves in water, then which of the following would be observed:
Wood spirit is known as acetone:
Write the IUPAC name of the following compound.
\[\begin{array}{cc}
\phantom{.}\ce{CH3 - CH - CH - CH - CH - CH3}\phantom{}\\
\phantom{......}|\phantom{......}|\phantom{......}|\phantom{.....}|\phantom{........}\\
\phantom{...}\ce{CH3}\phantom{...}\ce{OH}\phantom{...}\ce{C2H5}\phantom{.}\ce{OH}\phantom{...}\end{array}\]
Name the enzymes and write the reactions involved in the preparation of ethanol from sucrose by fermentation.
Which reagent can convert acetic acid into ethanol?
If the starting material is 1-methyl-1,2-epoxy cyclopentane, of absolute configuration, decide which one compound correctly represents the product of its reaction with sodium methoxide in methanol.
Convert the following Ethanal to ethanol.
Give IUPAC names of the following compounds:
\[\begin{array}{cc}
\ce{CH3 - CH - CH - CH - CH2-OH }\\
|\phantom{......}|\phantom{......}|\phantom{.......}\\
\ce{Cl}\phantom{....}\ce{CH3}\phantom{...}\ce{CH3}\phantom{.....}
\end{array}\]
