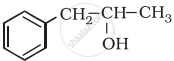Advertisements
Advertisements
Question
Why ethanol has the higher boiling point than ethane?
Solution
1) Alcohol has higher boiling points than their corresponding alkanes due to the presence of intermolecular hydrogen bonding which is absent in the alkanes.
2) Hydrogen bonding arises due to the presence of electronegative atom oxygen in –OH group of the alcohol.
3) Oxygen atom attracts electron density of O–H bond towards itself and hence it gets the partial negative charge, while H atom gets partial positive change.
`-delta + delta`
R-O-H
4) Hence in alcohol, R–OH molecules become polar
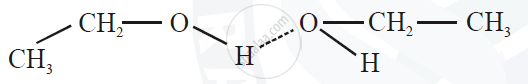
5) There arises strong intermolecular attraction between the oxygen atom of one molecule of alcohol and ‘H’ atom of another alcohol molecule, giving rise to strong hydrogen bonding which is not present in alkanes.
6) Hence higher thermal energy is required to separate or evaporate alcohol molecule. Therefore alcohols have higher boiling points than their corresponding alkanes.
APPEARS IN
RELATED QUESTIONS
Which of the followings is a trihydric alcohol ?
(a) n-propyl alcohol
(b) Glycerol
(c) Glycol
(d) Glycine
Identify allylic alcohols in the following examples.
Give the structures and IUPAC names of monohydric phenols of molecular formula C7H8O.
Write a chemical equation for the action of neutral ferric chloride on phenol.
Write balanced chemical equation for the following :
Action of sodium metal on ethanol.
How many alcohol(s) with molecular formula \[\ce{C4H10O}\] is chiral in nature?
Number of metamers represented by molecular formula C4H10O is ____________.
Benzyl alcohol is obtained from benzaldehyde by:
Among the following compounds, the strongest acid is:
How much ethyl alcohol must be added to 1 litre of water so that the solution will freeze at -14°C? (Kf for water = 1.86°C/mol)
Arrange the following in the increasing order of their property indicated:
Benzoic acid, Phenol, Picric acid, Salicylic acid (pka values).
\[\ce{n-Butane ->[AlCl3][HCl] [X] ->[KMnO4] [Y], [Y]}\] is
Distinction b/w primary, secondary and tertiary alcohol is done by:-
Glycerol is not used in which of the following cares
Main constituent of dynamite is.
Which is used an antifreeze
Washing soap can be prepared by saponification with alkali of the oil.
Explain the classification of alcohol and phenol.
Write IUPAC names of the following compound:
\[\begin{array}{cc}
\phantom{..............}\ce{CH3}\\
\phantom{............}|\\
\ce{CH3 - CH - CH - C - CH3}\\
|\phantom{......}|\phantom{......}|\\
\phantom{...}\ce{CH3}\phantom{...}\ce{OH}\phantom{...}\ce{CH3}
\end{array}\]
Write IUPAC names of the following compounds:
\[\begin{array}{cc}
\phantom{...............}\ce{CH3}\\
\phantom{.............}|\\
\ce{CH3 - CH - CH - C - CH3}\\
|\phantom{......}|\phantom{......}|\\
\phantom{...}\ce{CH3 \phantom{..}OH \phantom{..}CH3}
\end{array}\]
Classify the following as primary, secondary and tertiary alcohol:
H2C = CH – CH2OH
Classify the following as primary, secondary and tertiary alcohol:
CH3 – CH2 – CH2 – OH
Classify the following as primary, secondary and tertiary alcohol: