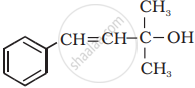Advertisements
Advertisements
Question
Give the structures and IUPAC names of monohydric phenols of molecular formula C7H8O.
Solution
The given molecular formula is C7H8O. As it is given that it is a phenol, the basic structure will be: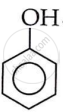
This structure accounts for C6H6O. When remaining CH2 is added to phenol, it forms the compounds shown below.
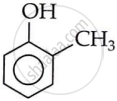
2-Methylphenol (o-cresol)
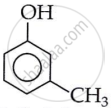
3-Methylphenol (m-cresol)
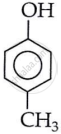
4-Methylphenol (p-cresol)
APPEARS IN
RELATED QUESTIONS
Classify the following as primary, secondary and tertiary alcohol:
\[\begin{array}{cc}
\ce{CH3}\phantom{.}\\
|\phantom{....}\\
\ce{CH3 - C - CH2OH}\\
|\phantom{....}\\
\ce{CH3}\phantom{.}
\end{array}\]
The alcohol used in thermometers is _______.
(A) methanol
(B) ethanol
(C) propanol
(D) butanol
Why ethanol has the higher boiling point than ethane?
Write a chemical equation for the action of neutral ferric chloride on phenol.
Write balanced chemical equation for the following :
Action of sodium metal on ethanol.
Which of the following is dihydric alcohol?
How many isomers of C5H11OH will be primary alcohols?
Number of metamers represented by molecular formula C4H10O is ____________.
Among the following compounds, the strongest acid is:
How much ethyl alcohol must be added to 1 litre of water so that the solution will freeze at -14°C? (Kf for water = 1.86°C/mol)
Arrange the following in the increasing order of their property indicated:
Benzoic acid, Phenol, Picric acid, Salicylic acid (pka values).
\[\ce{n-Butane ->[AlCl3][HCl] [X] ->[KMnO4] [Y], [Y]}\] is
Glycerol is not used in which of the following cares
Which is used an antifreeze
Washing soap can be prepared by saponification with alkali of the oil.
Classify the following as primary, secondary and tertiary alcohol: