Advertisements
Advertisements
Question
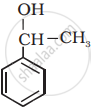
How many alcohol(s) with molecular formula \[\ce{C4H10O}\] is chiral in nature?
Options
1
2
3
4
Solution
1
Explanation:
\[\begin{array}{cc}
\ce{CH3-\overset{∗}{C}H-CH2-CH3}\\
|\phantom{........}\\
\ce{OH}\phantom{......}
\end{array}\]
has chiral centre (one).
APPEARS IN
RELATED QUESTIONS
Classify the following as primary, secondary and tertiary alcohol:
\[\begin{array}{cc}
\ce{CH3}\phantom{.}\\
|\phantom{....}\\
\ce{CH3 - C - CH2OH}\\
|\phantom{....}\\
\ce{CH3}\phantom{.}
\end{array}\]
Write a chemical equation for the action of neutral ferric chloride on phenol.
Write balanced chemical equation for the following :
Action of sodium metal on ethanol.
Among the following compounds, the strongest acid is:
Lower molecular mass alcohols are ______.
Which of the following compounds gives a secondary alcohol upon reaction with methylmagnesium bromide?
Distinction b/w primary, secondary and tertiary alcohol is done by:-
Explain the classification of alcohol and phenol.
Write IUPAC names of the following compound:
\[\begin{array}{cc}
\phantom{..............}\ce{CH3}\\
\phantom{............}|\\
\ce{CH3 - CH - CH - C - CH3}\\
|\phantom{......}|\phantom{......}|\\
\phantom{...}\ce{CH3}\phantom{...}\ce{OH}\phantom{...}\ce{CH3}
\end{array}\]
Classify the following as primary, secondary and tertiary alcohol: