Advertisements
Advertisements
Question
The conductivity of sodium chloride at 298 K has been determined at different concentrations and the results are given below:
| Concentration/M | 0.001 | 0.010 | 0.020 | 0.050 | 0.100 |
| 102 × κ/S m−1 | 1.237 | 11.85 | 23.15 | 55.53 | 106.74 |
Calculate `∧_"m"`for all concentrations and draw a plot between `∧_"m"`and `"c"^(1/2)`. Find the value of `∧_"m"^0`.
Solution
| Concentration (M) | C1/2 (M1/2) |
κ(S m−1) | κ(S cm−1) | `∧_"m" = (κ xx 1000)/"M"` (S cm2 mol−1) |
| 0.001 | 0.0316 | 1.237 × 10−2 | 1.237 × 10−4 | `∧_"m" = (1.237 xx 10^-4 xx 1000)/0.001` = 123.7 |
| 0.010 | 0.100 | 11.85 × 10−2 | 11.85 × 10−4 | `∧_"m" = (11.85 xx 10^-4 xx 1000)/(0.010)` = 118.5 |
| 0.020 | 0.141 | 23.15 × 10−2 | 23.15 × 10−4 | `∧_"m" = (23.15 xx 10^-4 xx 1000)/0.020` = 115.8 |
| 0.050 | 0.224 | 55.53 × 10−2 | 55.53 × 10−4 | `∧_"m" = (55.53 xx 10^-4 xx 1000)/(0.1050)` = 111.1 |
| 0.100 | 0.316 | 106.74 × 10−2 | 106.74 × 10−4 | `∧_"m" = (106.74 xx 10^-4 xx 1000)/(0.100)` = 106.7 |
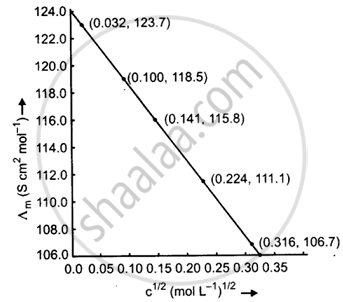
When the straight line is drawn backwards, it meets the `∧_"m"`axis at 124.0 S cm2 mol−1, which is the value of `∧_"m"^0`.
APPEARS IN
RELATED QUESTIONS
State Kohlrausch’s law of independent migration of ions.
Define the following terms: Molar conductivity (⋀m)
The molar conductivity of 0.025 mol L−1 methanoic acid is 46.1 S cm2 mol−1. Calculate its degree of dissociation and dissociation constant. Given \[\ce{λ^0_{(H^+)}}\] = 349.6 S cm2 mol−1 and \[\ce{λ^0_{(HCOO^-)}}\] = 54.6 S cm2 mol−1.
10.0 grams of caustic soda when dissolved in 250 cm3 of water, the resultant gram molarity of solution is _______.
(A) 0.25 M
(B) 0.5 M
(C) 1.0 M
(D) 0.1 M
The conductivity of 0.02M AgNO3 at 25°C is 2.428 x 10-3 Ω-1 cm-1. What is its molar
conductivity?
A steady current of 2 amperes was passed through two electrolytic cells X and Y connected in series containing electrolytes FeSO4and ZnSO4 until 2.8g of Fe deposited at the cathode of cell X. How long did the current flow? Calculate the mass of Zn deposited at the cathode of cell Y.
(Molar mass: Fe=56g mol-1,Zn=65.3g mol-1,1F=96500C mol-1)
In the plot of molar conductivity (∧m) vs square root of concentration (c1/2), following curves are obtained for two electrolytes A and B:
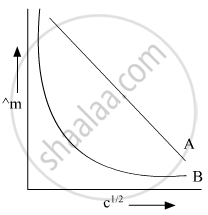
Answer the following:
(i) Predict the nature of electrolytes A and B.
(ii) What happens on extrapolation of ∧m to concentration approaching zero for electrolytes A and B?
Conductivity always decreases with decrease in concentration both, for weak and strong electrolytes because of the fact that ____________.
\[\ce{Λ^0_m H2O}\] is equal to:
(i) \[\ce{Λ^0_m_{(HCl)} + \ce{Λ^0_m_{(NaOH)} - \ce{Λ^0_m_{(NaCl)}}}}\]
(ii) \[\ce{Λ^0_m_{(HNO_3)} + \ce{Λ^0_m_{(NaNO_3)} - \ce{Λ^0_m_{(NaOH)}}}}\]
(iii) \[\ce{Λ^0_{(HNO_3)} + \ce{Λ^0_m_{(NaOH)} - \ce{Λ^0_m_{(NaNO_3)}}}}\]
(iv) \[\ce{Λ^0_m_{(NH_4OH)} + \ce{Λ^0_m_{(HCl)} - \ce{Λ^0_m_{(NH_4Cl)}}}}\]
Write the cell reaction of a lead storage battery when it is discharged. How does the density of the electrolyte change when the battery is discharged?
Why on dilution the m Λm of \[\ce{CH3COOH}\] increases very fast, while that of \[\ce{CH3COONa}\] increases gradually?
Assertion: Λm for weak electrolytes shows a sharp increase when the electrolytic solution is diluted.
Reason: For weak electrolytes degree of dissociation increases with dilution of solution.
Assertion: Copper sulphate can be stored in zinc vessel.
Reason: Zinc is less reactive than copper.
Which of the following increases with the increase in the concentration of the solution?
The molar conductivity of 0.007 M acetic acid is 20 S cm2 mol−1. What is the dissociation constant of acetic acid? Choose the correct option.
`[(Λ_("H"^+)^ο = 350 "S" "cm"^2 "mol"^-1), (Λ_("CH"_3"COO"^-)^ο = 50 "S" "cm"^2 "mol"^-1)]`
Given below are two statements:
Statements I: The limiting molar conductivity of KCl (strong electrolyte) is higher compared to that of CH3COOH (weak electrolyte).
Statement II: Molar conductivity decreases with decrease in concentration of electrolyte.
In the light of the above statements, choose the most appropriate answer from the options given below:
The resistance of a conductivity cell with a 0.1 M KCl solution is 200 ohm. When the same cell is filled with a 0.02 M NaCl solution, the resistance is 1100 ohm. If the conductivity of 0.1 M KCl solution is 0.0129 ohm-1 cm-1, calculate the cell constant and molar conductivity of 0.02 M NaCl solution.
The solution of two electrolytes A and B are diluted. ^m of B increases 1.5 times while that of A increases 25 times. Which of the two is a strong electrolyte? Give a reason.
