Advertisements
Advertisements
Question
Assertion: Copper sulphate can be stored in zinc vessel.
Reason: Zinc is less reactive than copper.
Options
Both assertion and reason are true and the reason is the correct explanation of assertion.
Both assertion and reason are true and the reason is not the correct explanation of assertion.
Assertion is true but the reason is false.
Both assertion and reason are false.
Assertion is false but reason is true.
Solution
Both assertion and reason are false.
Explanation:
Zinc will get dissolved in CuS04 solution since zinc is more reactive than copper.
APPEARS IN
RELATED QUESTIONS
The molar conductivity of 0.025 mol L−1 methanoic acid is 46.1 S cm2 mol−1. Calculate its degree of dissociation and dissociation constant. Given \[\ce{λ^0_{(H^+)}}\] = 349.6 S cm2 mol−1 and \[\ce{λ^0_{(HCOO^-)}}\] = 54.6 S cm2 mol−1.
The conductivity of sodium chloride at 298 K has been determined at different concentrations and the results are given below:
| Concentration/M | 0.001 | 0.010 | 0.020 | 0.050 | 0.100 |
| 102 × κ/S m−1 | 1.237 | 11.85 | 23.15 | 55.53 | 106.74 |
Calculate `∧_"m"`for all concentrations and draw a plot between `∧_"m"`and `"c"^(1/2)`. Find the value of `∧_"m"^0`.
10.0 grams of caustic soda when dissolved in 250 cm3 of water, the resultant gram molarity of solution is _______.
(A) 0.25 M
(B) 0.5 M
(C) 1.0 M
(D) 0.1 M
Molar conductivity denoted by the symbol Λm is related to the conductivity of the solution by the equation (k is the conductivity and c is the concentration).
Which of the statements about solutions of electrolytes is not correct?
\[\ce{Λ^0_m}_{(NH_4OH)}\] is equal to ______.
Write the cell reaction of a lead storage battery when it is discharged. How does the density of the electrolyte change when the battery is discharged?
Consider figure and answer the question to given below.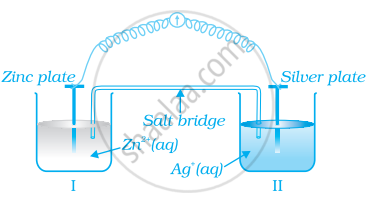
How will the concentration of Zn2+ ions and Ag+ ions be affected after the cell becomes ‘dead’?
Which of the following halogen acids is the strongest reducing agent?
Suggest a way to determine the `∧_"m"^∘`value of water.
