Advertisements
Advertisements
Question
The difference between the electrode potentials of two electrodes when no current is drawn through the cell is called ______.
Options
Cell potential
Cell emf
Potential difference
Cell voltage
Solution
The difference between the electrode potentials of two electrodes when no current is drawn through the cell is called cell emf.
Explanation:
EMF is the difference between the electrode potentials of two electrodes • when no current is drawn through the cell.
APPEARS IN
RELATED QUESTIONS
Draw a neat and well labelled diagram of primary reference electrode.
Calculate Ecell and ΔG for the following at 28°C :
Mg(s) + Sn2+( 0.04M ) → Mg2+( 0.06M ) + Sn(s)
E°cell = 2.23V. Is the reaction spontaneous ?
Depict the galvanic cell in which the reaction \[\ce{Zn(s) + 2Ag+(aq) → Zn^{2+}(aq) + 2Ag(s)}\] takes place. Further show:
- Which of the electrode is negatively charged?
- The carriers of the current in the cell.
- Individual reaction at each electrode.
In the representation of the galvanic cell, the ions in the same phase are separated by a _______.
Draw a neat and labelled diagram of the lead storage battery.
Galvanic or a voltaic cell converts the chemical energy liberated during a redox reaction to ____________.
Using the data given below find out the strongest reducing agent.
`"E"_("Cr"_2"O"_7^(2-)//"Cr"^(3+))^⊖` = 1.33 V `"E"_("Cl"_2//"Cl"^-) = 1.36` V
`"E"_("MnO"_4^-//"Mn"^(2+))` = 1.51 V `"E"_("Cr"^(3+)//"Cr")` = - 0.74 V
Consider the figure and answer the following question.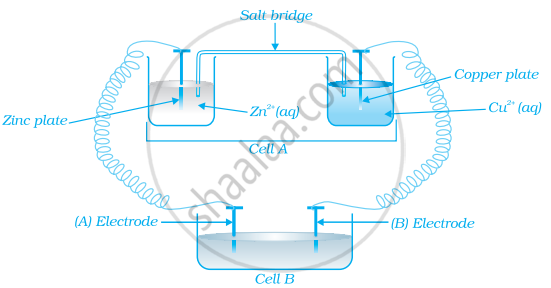
If cell ‘A’ has ECell = 0.5V and cell ‘B’ has ECell = 1.1V then what will be the reactions at anode and cathode?
Standard electrode potential of three metals X, Y and Z are – 1.2 V, + 0.5 V and – 3.0 V, respectively. The reducing power of these metals will be:
The potential of a hydrogen electrode at PH = 10 is
