Advertisements
Advertisements
Question
For the given cell, \[\ce{Mg | Mg^{2+} || Cu^{2+} | Cu}\]
(i) \[\ce{Mg}\] is cathode
(ii) \[\ce{Cu}\] is cathode
(iii) The cell reaction is \[\ce{Mg^+ Cu^{2+} -> Mg^{2+} + Cu}\]
(iv) \[\ce{Cu}\] is the oxidising agent
Solution
(ii) \[\ce{Cu}\] is cathode
(iii) The cell reaction is \[\ce{Mg^+ Cu^{2+} -> Mg^{2+} + Cu}\]
Explanation:
(i) Left side of cell reaction represents oxidation half-cell i.e., oxidation of \[\ce{Mg}\] and right side of cell represents reduction half-cell reactions i.e., reduction of copper.
(ii) \[\ce{Cu}\] is reduced and reduction occurs at cathode.
(iii) \[\ce{Mg}\] is oxidized and oxidation occurs at anode.
(iv) Whole cell reaction can be written as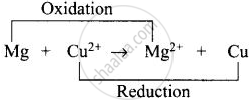
APPEARS IN
RELATED QUESTIONS
What happens if external potential applied becomes greater than E°cell of electrochemical cell?
A solution of Cu(NO3)2 is electrolyzed between platinum electrodes using 0.1 Faraday electricity. How many moles of Cu will be deposited at the cathode?
Zinc can be coated on iron to produce galvanized iron but the reverse is not possible. It is because ____________.
In the electrochemical cell: Zn|ZnSO4 (0.01 M)||CuSO4 (1.0 M)|Cu, the emf of this Daniel cell is E1. When the concentration of ZnSO4 is changed to 1.0 M and that CuSO4 changed to 0.01 M, the emf changes to E2. From the above, which one is the relationship between E1 and E2?
Why is anode in galvanic cell considered to be negative and cathode positive electrode?
Can Fe3+ oxidises bromide to bromine under standard conditions?
Given: \[\ce{E^0_{{Fe^{3+}|Fe^{2+}}}}\] = 0.771 V
\[\ce{E^0_{{Br_{2}|Br^-}}}\] = −1.09 V
The quantity of charge required to obtain one mole of aluminium from Al2O3 is ______.
Match the items of Column I and Column II.
| Column I | Column II |
| (i) K | (a) I × t |
| (ii) Λm | (b) `Λ_m/Λ_m^0` |
| (iii) α | (c) `K/c` |
| (iv) Q | (d) `G^∗/R` |
If the value of Ksp for Hg2Cl2 (s) is X then the value of X will be ____ where pX = - log X.
Given:
\[\ce{Hg2Cl2 + 2e- -> 2Hg(l) + 2Cl-}\], E° = 0.27 V
\[\ce{Hg+2 + 2e- -> 2Hg(l)}\] E° = 0.81 V
Explain the types of electrochemical cells.
